Introduction to blood.
Basic descriptions of blood cell types.
See the Elektronenmikroskopischer Atlas im Internet for (mostly unlabelled) EM images of blood cells.
Examples from peripheral blood smears.
How to look at blood smears.
Red blood cells (abbreviated RBCs, also called erythrocytes (from erythro = red + cyte = cell) are continually produced in bone marrow and recycled in spleen. In mature form they lack nuclei and most cytoplasmic structures; they are little more than discoid, flexible bags of hemoglobin.
Hematopathology: Examples of normal and abnormal RBCs from Webpath.
RBCs in other animals. RBCs retain their nuclei in non-mammalian vertebrates: fish, birds, reptiles and amphibians. However, in all mammals including humans, mature RBCs extrude their nuclei. Hence, in all mammals, circulating RBCs are without nuclei.
A persistent misconception (easily found on the internet, also in the prominent textbook Mammalogy) holds that camels are an exception to this rule. The false claim is that camels, unlike all other mammals, do have nuclei in their RBCs. Camelid RBCs (including llamas etc. as well as camels) are indeed peculiar. Unlike the biconcave discs of typical mammalian RBCs, camel RBCs are shaped somewhat like rugby balls, thickest in the center. And they are extremely resistant to substantial changes in osmotic pressure. Nevertheless, as can be easily confirmed from readily available images (e.g., here), camel RBCs do not have nuclei.
Historical note: The misconception that camel RBCs have nuclei was introduced in 1877, but the scientific literature has recognized this as an error for nearly a century:
1928, Ponder E, Yeager JF, and Charipper HA. Studies in Comparative Haematology: I. Camelidae. Quarterly Journal of Experimental Physiology 19.2 pp 115-126: "When the cells are fixed in methyl alcohol, the haemoglobin is especially deposited in the central parts of the cell, which, as a result, take on a somewhat deeper stain than the peripheral areas. This appearance may possibly be responsible for the erroneous statement which has sometimes been made that the cells are nucleated [Böttcher, Arch. f. mikr. Anat., 1877, xiv. 73]" [emphasis added].
[Ponder et al. (above) are correct in attributing to Boettcher an "erroneous statement" that camel RBCs have nuclei. But they are perhaps a bit unfair in suggesting that Boettcher, an accomplished histologist, might have made such a simple mistake solely on the basis of cell thickness. In his 1877 publication, Boettcher acknowledges that prior researchers had failed to find nuclei in camel RBCs, and he notes that they are indeed difficult to observe. But he goes on to describe detailed observations, after manipulation of fixation and stain protocols, of a clear distinction between different regions within the cells' contents, which he then interprets as indicative of the presence of nuclei. What Boettcher lacked was an effective and trustworthy stain for chromatin (a word which, incidently, was not coined until 1879 by Walther Flemming).]Unfortunately, the same "erroneous statement" has been perpetuated ever since, notably in the otherwise-outstanding textbook, Mammalogy, by Vaughan, Ryan & Czaplewski, Saunders College Publishing. Here is a quote from the 5th edition, 2005: "In all mammals except camels (Camelidae), the erythrocytes extrude their nuclei..." [emphasis added]. This error continues, in spite of being noted by The Journal of Mammology (vol. 81, p. 916; 2000) in a review of the 4th edition of this textbook: "The authors follow a long-perpetuated error in science, that camels have nucleate red blood cells" [emphasis added].
White blood cells (abbreviated WBCs, also called leukocytes from leuko = white + cyte = cell) comprise several distinct cell types, neutrophils, eosinophils, basophils, lymphocytes and monocytes. Certain developmental and morphological similarities permit the first three these cells to be usefully grouped together as granulocytes or polymorphonuclear leukocytes. The latter two types are then categorized as mononuclear leukocytes.
White blood cells are called white blood cells because of their intrinsic color, in contrast to red blood cells, which are red from hemoglobin. In blood fractionated by centrifugation, plasma forms a clear layer, WBCs form a creamy-colored layer, and RBCs form a red layer.
Granulocytes, also called polymorphonuclear leukocytes, have grainy cytoplasm and elongated or lobed nuclei. They arise in bone marrow from cells called myeloblasts and pass through stages called myelocytes and metamyelocytes. The granulocytes include neutrophils, eosinophils, and basophils.
Neutrophils (also called neutrophilic granulocytes, or polymorphonuclear neutrophilic leukocytes, PMNs, or polys) are the most numerous of the leukocytes, about 60% of the white blood cell count. They are about 12 Ám in diameter in blood smear preparations (about twice the size of red blood cells).
The condition of having too many neutrophils is called neutrophilia.
Neutrophils take their name from the staining properties of their cytoplasmic lysosomal granules (vesicles containing stored lysosomal enzymes). These granules are neutrophilic, meaning they show no special affinity for either acidic or basic stains but are stained mildly by both. (This is in contrast to the specific granules of eosinophils, which stain red with acidic stains such as eosin, and those of basophils, which stain with basic stains.)
The nuclei of mature neutrophils are elongated and pinched into several distinct lobes, hence the term polymorphonuclear. This characteristic nuclear structure confers a distinctive appearance on neutrophils, both in blood smears and in tissue sections. Immature neutrophils have a band-shaped nucleus and are hence sometimes called "bands." Mature neutrophils, in contrast, are called "segs," in reference to the segmented nucleus.
Neutrophils are anti-bacterial cells which lyse (break down) bacterial cells by releasing the lysosomal enzymes which are stored in their specific granules. Neutrophils gather rapidly in peripheral tissue during acute inflammation, by emigrating from the blood (Webpath). There they recognize bacteria as foreign by the antibodies which have attached to the bacterial surface. [Antibodies are molecules found in blood plasma and interstitial fluid which bind to specific foreign antigens.]
Neutrophils are only occasionally seen in normal tissue sections outside blood (except, of course, in inflamed tissue). Here they may be most easily recognized by their lobed nuclei. One neutrophil nucleus might be mistaken for a cluster of very small nuclei, but each of the lobes is much too small to be an entire nucleus -- only two or three Ám across, much smaller than the nuclei of lymphocytes which are among the smallest of our cells.
Eosinophils (eosinophilic granulocytes) normally comprise less than two to four percent of the peripheral leukocytes. Their specific granules are intense eosinophilic (stained by eosin), hence the name. Eosinophils are about the same size as neutrophils. Their nuclei are typically band shaped (elongated) or two-lobed.
The function of eosinophils remains obscure, although they are known to proliferate in association with allergies and parasites.
Basophils (basophilic granulocytes) normally comprise less than 1 % of the peripheral leukocytes. Their specific granules are intense basophilic, hence the name. Like eosinophils, basophils are similar in size to neutrophils. Their nuclei may be band shaped or segmented.
Basophils seem to be functionally similar to tissue mast cells, involved in triggering inflammation.
Mononuclear leukocytes comprise both lymphocytes and monocytes. Both cell types work together in immune responses.
Monocytes are the largest of the leukocytes, and constitute about 5 % of the WBC population in peripheral blood. In blood smears, their nuclei are typically indented, sometimes deeply so, with a kidney-bean or bent-horseshoe shape.
Monocytes belong to the same functional population as tissue macrophages. Monocytes/macrophages engulf and digest foreign microorganisms, dead or worn-out cells, and other tissue debris. They interact closely with lymphocytes to recognize and destroy foreign substances. Most ordinary connective tissues contain resident macrophages which normally remain at rest in the tissue. But the normal number of fixed macrophages is supplemented during inflammation by the influx of many monocytes from the blood.
Lymphocytes are small cells, 7-9 Ám in diameter in blood smears, and are the second most common white
blood cell type, comprising about 30 % of the leukocyte population in peripheral blood. Lymphocytes have a round heterochromatic (deeply staining) nucleus surrounded by a relatively thin rim of cytoplasm.
Lymphocytes travel in the blood, but they routinely leave capillaries and wander through connective tissue. Therefore, lymphocytes may be normally encountered at any time in any location. They even enter epithelial tissue, crawling between the epithelial cells. They reenter circulation via lymphatic system channels (hence their name).
Lymphocytes also emigrate from blood in response to inflammation, but they accumulate somewhat later during the inflammatory process than neutrophils. Their presence in large numbers indicates the continuing presence of antigen. Lymphocytes produce the multitude of diverse antibody molecules (one specific type of antibody per lymphocyte) which provide the mechanism for chemical recognition of foreign materials (distinguishing between self and non-self) and so for mediating and regulating immune responses.
Lymphocytes are most easily recognized in histological sections as small round "naked" nuclei (the cytoplasm is usually inconspicuous) which occur here and there in most connective tissues, especially commonly near mucous membranes. Lymphocytes are found densely packed in lymphoid tissue--spleen, lymph nodes, and lymph nodules in mucous membranes (e.g., tonsils, appendix), where they proliferate.
Plasma cells are lymphocytes which are specialized for mass production and secretion of circulating antibodies. Plasma cells have more extensive cytoplasm filled with rough endoplasmic reticulum (for synthesizing protein, specifically antibody molecules). This cytoplasm is distinctly basophilic, a consequence of the large numbers of ribosomes associated with the rER, and typically forms a lopsided bulge on one side of the nucleus. The heterochromatin of plasma cells is typically clumped in a characteristic "spoke-wheel" arrangement which also aids plasma cell recognition.
For more about the immune system see CRR Lymphatic System.
Platelets are small fragments of cytoplasm derived from bone marrow cells called megakaryocytes (mega = large + karyon = nucleus + cyte = cell). Among the confusing proliferation of haemopoietic cells in bone marrow, megakaryocytes are easy to recognize by their huge size and large, lobulated nuclei. Small bits of megakaryocyte cytoplasm are continually pinched off to enter circulation as platelets, where they are important for blood clotting.
The mechanism of platelet formation involves thin processes ("proplatelets") which megakaryocytes extend across bone-marrow endothelium into the blood flow. Shear forces in the blood tear small bits off the proplatelet processes, which may then fragment further in the lungs to form platelets. See Science 317:1689 (21 Sept. 2007).
Examples of white blood cells from peripheral blood smears
| Normal (?) Peripheral Blood |
Sickle Cell |
| Image 1 / Image
2 / Image 3 Image 4 / Image 5 / Image 6 Image 7 / Image 8 |
Image 1 / Image
2 / Image 3 Image 4 / Image 5 / Image 6 |
Dragging the scroll bar will enable rapid scanning of all images.
Note how easily you can determine when the images shift from the normal blood smear to the sickle-cell blood smear.
In a sickle-cell smear, many RBCs have irregular, flattened shapes (i.e., "sickle-cells") and some may have dark centers ("target cells").
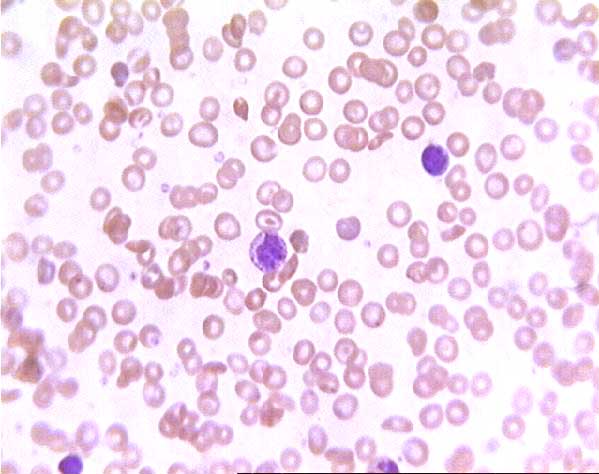
Normal (?) blood smear. Click on any white blood cell to see its name.
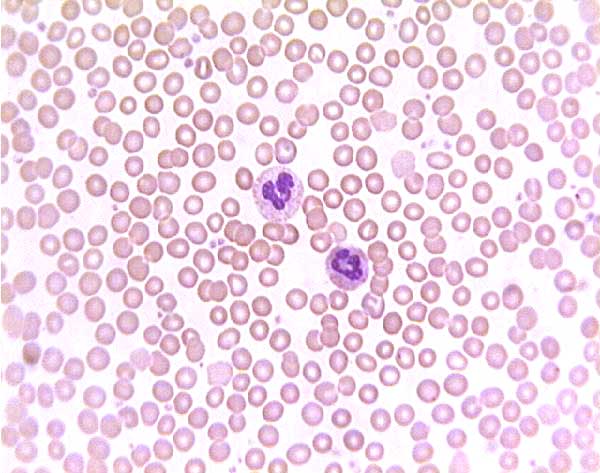
Normal (?) blood smear. Click on any white blood cell to see its name.
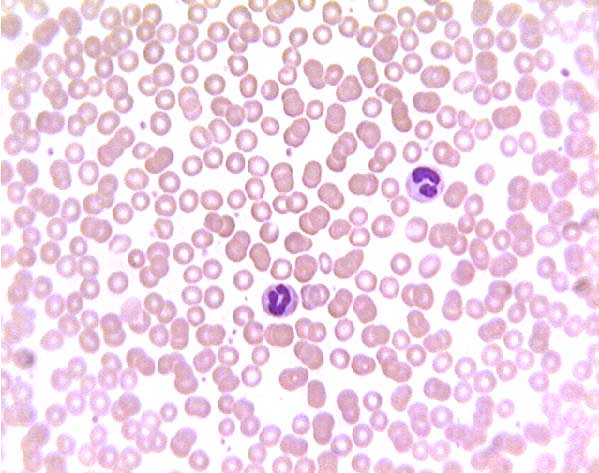
Normal (?) blood smear. Click on any white blood cell to see its name.
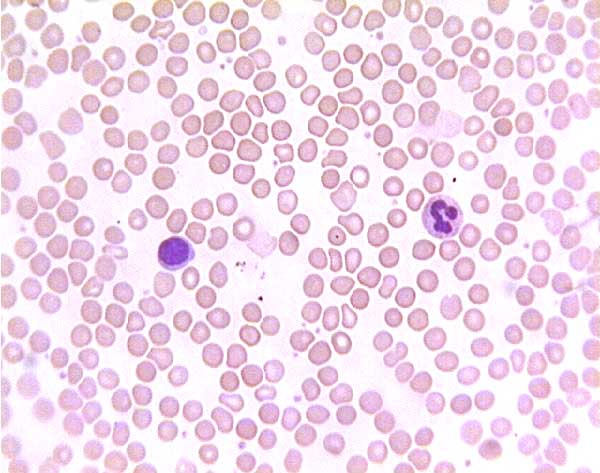
Normal (?) blood smear. Click on any white blood cell to see its name.
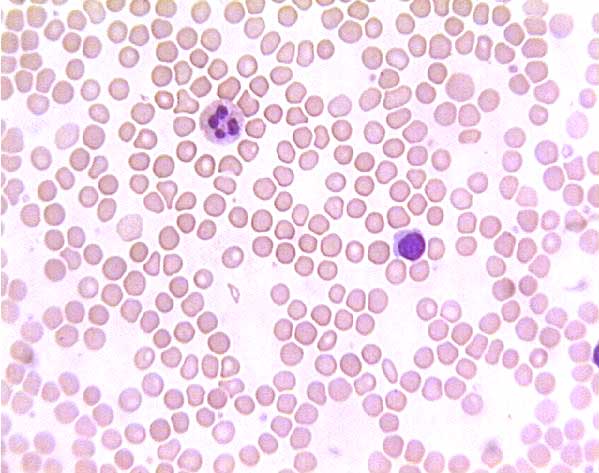
Normal (?) blood smear. Click on any white blood cell to see its name.
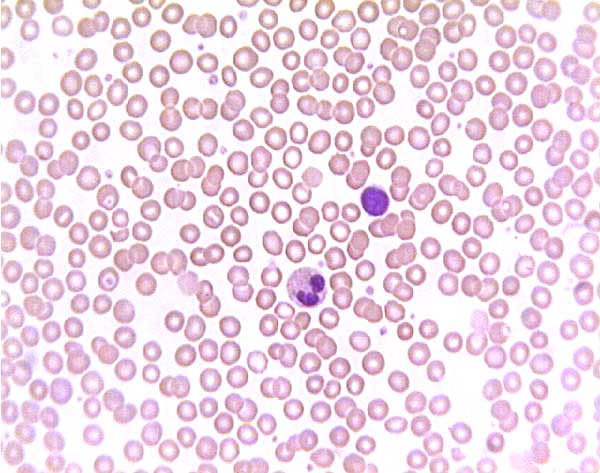
Normal (?) blood smear. Click on any white blood cell to see its name.
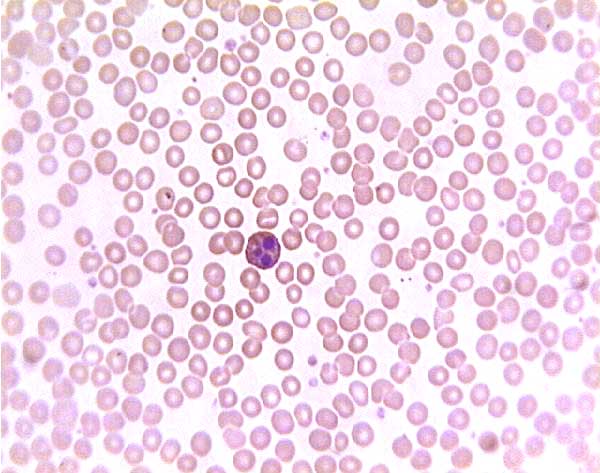
Normal (?) blood smear. Click on any white blood cell to see its name.
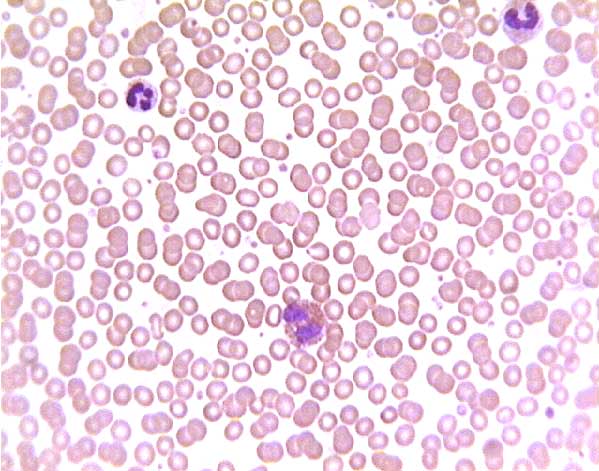
Normal (?) blood smear. Click on any white blood cell to see its name.
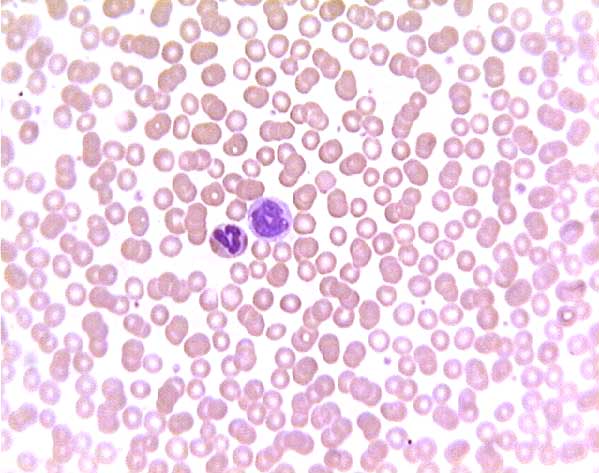
Sickle cell blood smear. Click on any white blood cell to see its name.
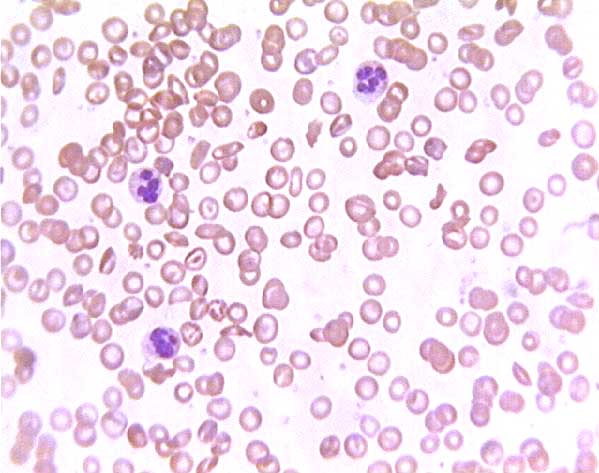
Sickle cell blood smear. Click on any white blood cell to see its name.
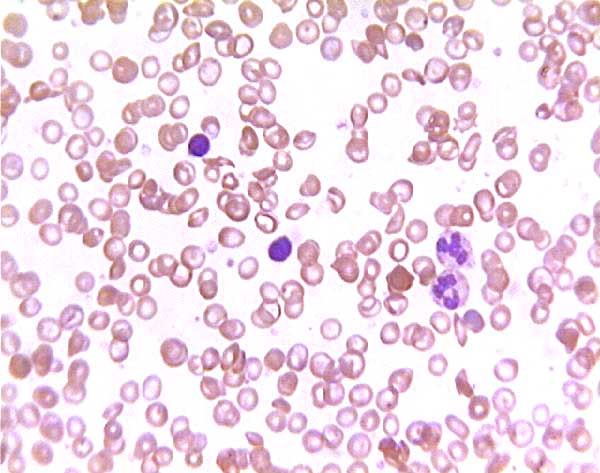
Sickle cell blood smear. Click on any white blood cell to see its name.
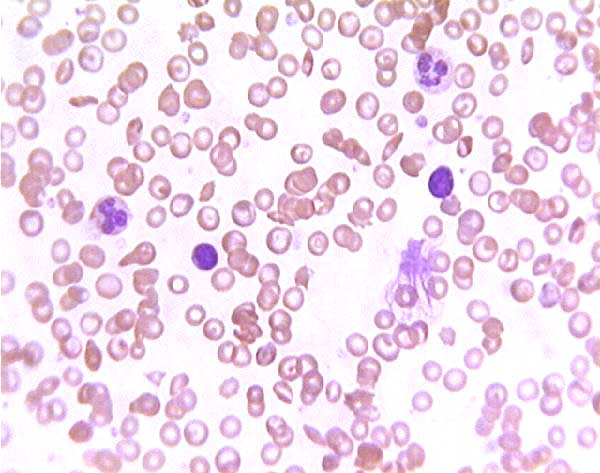
Sickle cell blood smear. Click on any white blood cell to see its name.
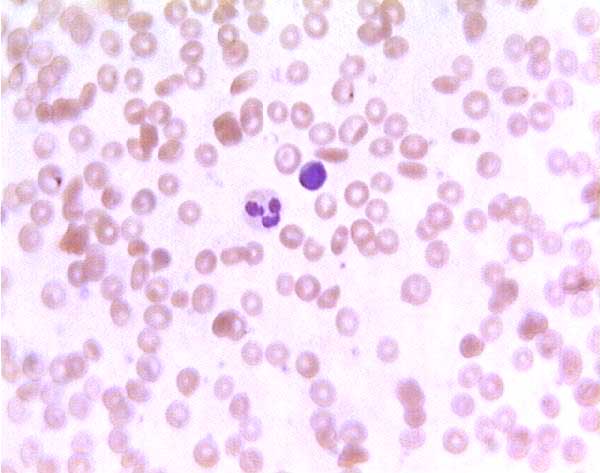
Sickle cell blood smear. Click on any white blood cell to see its name.
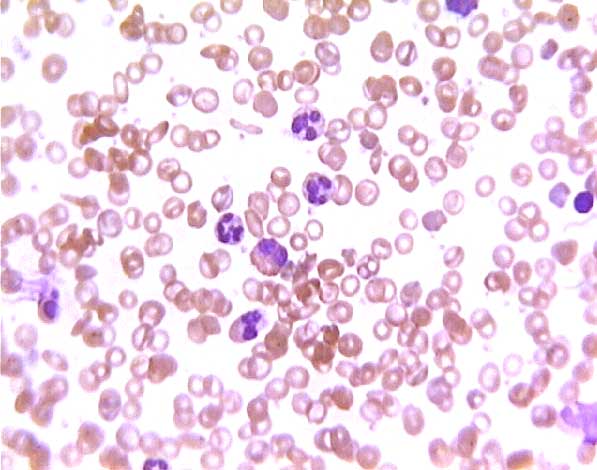
Sickle cell blood smear. Click on any white blood cell to see its name.
Comments and questions: dgking@siu.edu
SIUC / School
of Medicine / Anatomy / David
King
https://histology.siu.edu/intro/bldcells.htm
Last updated: 19 February 2024 / dgk
