
 Neurons
and Support Cells
Neurons
and Support Cells
Please note that this guide is intended to complement,
NOT to replace, textbook readings.
TOP OF PAGE
Histology textbooks are NOT recommended for the study of nervous tissue.
Rather than emphasizing features important for understanding nervous
tissue function, most histology textbooks begin with relatively insignificant, and often
misleading, details. (For example, many histology texts classify
dorsal root ganglion neurons as "pseudo-unipolar," which is
accurate but useless, since that that category is never mentioned again.
More significantly, the distal branch of such a cell's axon is not infrequently referred to as
a "dendrite," simply because it conducts toward the cell body. But by all generally
recognized criteria, this process is a sensory axon.)
RECOMMENDED are selected chapters in Kandel's Principles of Neural Science.
Kandel's classic text is remarkable. The extended table of contents
can be read, just as if it were a "capsule" textbook.
In about two dozen pages following immediately after the chapter listing,
all of the subheadings from every chapter are presented, each as a complete
sentence. This extended table of contents offers a concise summary
of major ideas. Your study through this entire unit can be usefully
guided by this summary. You should, at a minimum, be fluent in the
vocabulary of this summary so that every sentence here is meaningful to you.
Senior author Eric
Kandel shared the 2000 Nobel Prize
for Physiology and Medicine for work on "signal transduction in the nervous system"
(Kandel's Nobel Prize lecture;
Kandel's Nobel banquet speech;
Kandel extended biography).
- 6th edition, 2021, Kandel, Koester, Mack and Siegelbum
- Historical overview. Chapter 1 provides perspective
on how science and medicine have understood the brain.
- Basic cellular organization of the nervous system is described
in Chapter 3.
- Nerve cell structure and function are described in detail in
Chapters 7-10. Begin by reading the extended table of
contents.
- Glia, CSF, meninges, choroid plexus and blood brain barrier
are described toward the end of Ch. 7.
- 5th edition, 2012
- Historical overview. Chapter 1 provides an excellent
narrative of how science and medicine have understood the brain.
- Basic cellular organization of the nervous system is described
in Chapter 2.
- Nerve cell structure is described in detail in Chapter 4
and subsequent chapters. Begin by reading the extended table of
contents.
- [Appendix A reviews basic physics of electrical circuits, for
those who wish to go there.]
- CSF, meninges, choroid plexus and blood brain barrier
are described in Appendix D
- 4th edition, 2000
- Historical overview. Chapter 1 provides an excellent
narrative of how science and medicine have understood the brain.
- Basic cellular organization of the nervous system is described
in Chapter 2.
- Nerve cell structure is described in detail in Chapter 4.
Begin by reading the extended table of contents (p. x). Add
more only as the need arises.
- Subcellular basis for neural function. Begin with the extended
table of contents (pp. x-xi). Check out Chapters 3
(genes), Chapter 5 (proteins), and Chapters 6-9
(electrical activity). Dip into the main text for principal
learning issues (e.g., resting potential and action potential). [Appendix
A reviews basic physics of electrical circuits, for those who wish
to go there.]
- 3rd edition, 1991
- Historical overview. Chapter 1 provides an excellent
narrative of how science and medicine have understood the brain.
- Basic cellular organization of the nervous system is described
in Chapter 2.
- Nerve cell structure is described in detail in Chapter 3.
Begin by reading the extended table of contents (p. xiii).
Add more only as the need arises.
- Subcellular basis for neural function. Begin with the extended
table of contents (pp. xiv-xv). Check out Chapters
4 (proteins), and Chapters 5-8 (electrical
activity). Dip into the main text for principal learning issues
(e.g., resting potential and action potential). [Appendix A
reviews basic physics of electrical circuits, for those who wish to go
there.]
TOP OF PAGE
INTRODUCTORY COMMENTS
Please note that this guide is intended to complement,
NOT to replace, textbook readings (i.e., Kandel
et al.).
If you feel intimidated by heavy textbooks, you might appreciate a collection of
2-minute mini-lectures at NEUROSCIENTIFICALLYCHALLENGED.com.
The study of nervous tissue presents extraordinary challenges. Historically, other tissues were fairly well
understood decades before science acquired a basic appreciation for the cellular composition of nervous tissue.
One reason is that routine histological preparations do not enable proper visualization of any nerve cell type.
Significance for study: Do not expect microscopic examination of nervous system specimens to yield
satisfying observations, at least not without an exceptional effort to understand why things look the way they do.
☞ An excellent
New York
Times article, with video, describes in detail how brain tissue is processed for histology in a modern pathology laboratory.
(This article [Sept. 9, 2025] was written in the context of diagnosing chronic traumatic encephalopathy, C.T.E.
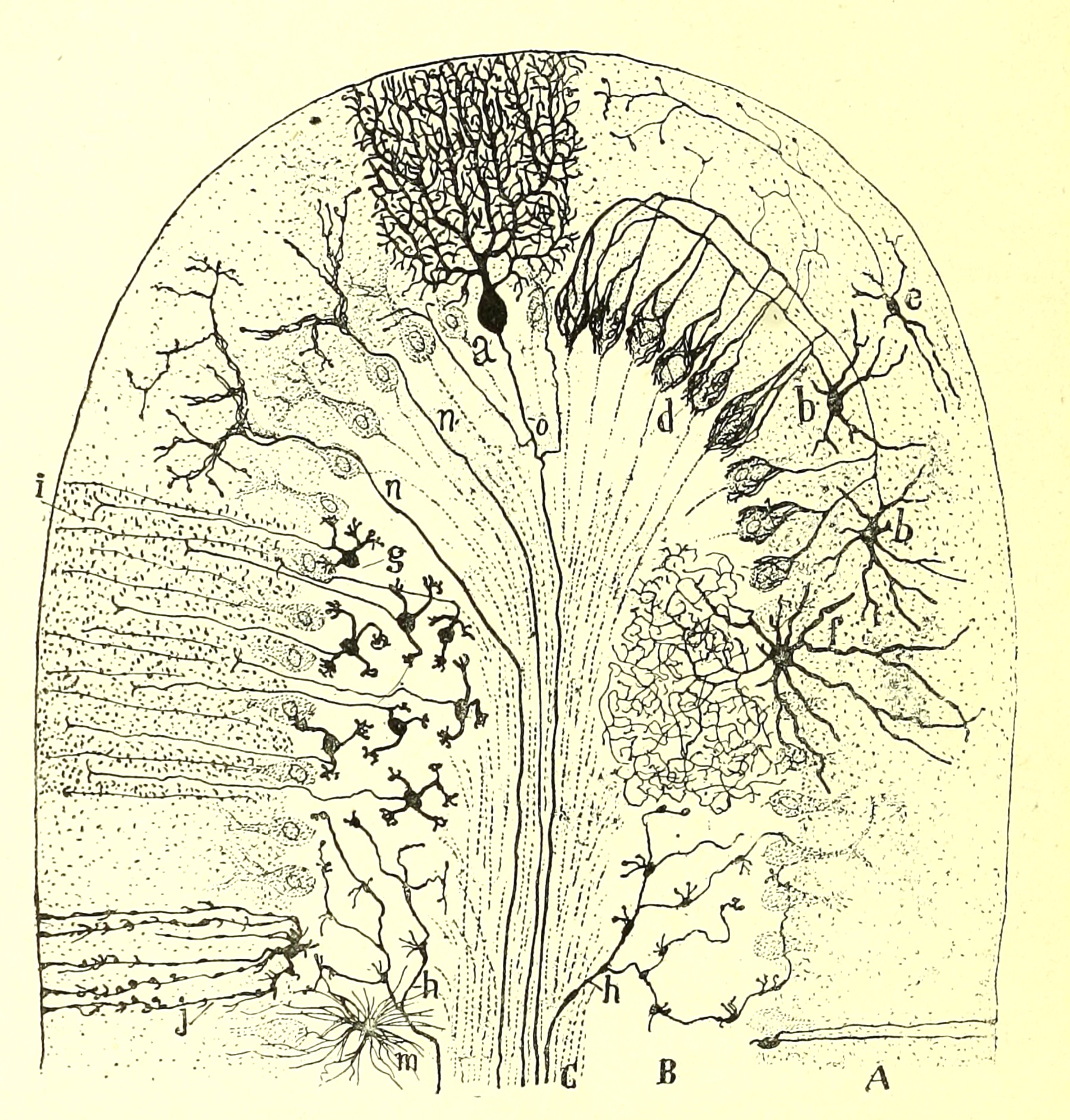
The Neuron Doctrine
Modern understanding of nervous tissue (i.e., since the early 20th century) is summarized by the
four principles of The Neuron Doctrine (quotations below are from Eric Kandel's 2006 autobiography
In Search of Memory, pp. 65-66):
Historical note: These four principles were introduced by Santiago
Ramón y Cajal, the most famous pioneer in
the descriptive anatomy of nerve cells. Cajal's
1906 Nobel Prize lecture includes some elegant images of nerve cells in spinal cord and in cerebellar and cerebral cortex.
(For curiosity, see "Milestones in Neuroscience Research," a lengthy
list covering several thousand years.)
- Cellularity: "The nerve cell is the fundamental
structural and functional element of the brain."
- Synaptic communication: "The terminals of
one neuron's axon communicate with the dendrites of another neuron only
at specialized sites, later named 'synapses' by
Sherrington."
- Connection specificity: "Neurons do not
form connections indiscriminately. Rather each nerve cell forms
synapses and communicates with certain nerve cells and not with others."
- Dynamic polarization: "Signals in a neural
circuit travel in only one direction... Information flows, from
the dendrites of a given nerve cell to the cell body [then] along the
axon to the presynaptic terminals and then across the synaptic cleft to
the dendrites of the next cell, and so on."
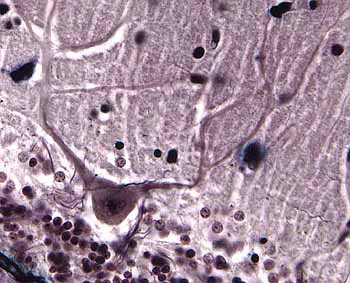
 Axons,
dendrites and synapses -- the most significant features of nerve
cells -- cannot be readily seen without specialized techniques such as
those used by Cajal. (The spaces between nerve cell bodies are filled
with a feltwork of these axonal and dendritic processes,
called neuropil, which also includes glial
cell processes.)
Axons,
dendrites and synapses -- the most significant features of nerve
cells -- cannot be readily seen without specialized techniques such as
those used by Cajal. (The spaces between nerve cell bodies are filled
with a feltwork of these axonal and dendritic processes,
called neuropil, which also includes glial
cell processes.)
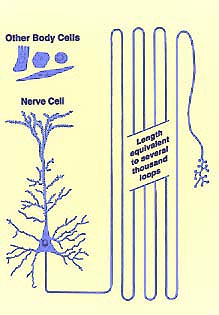
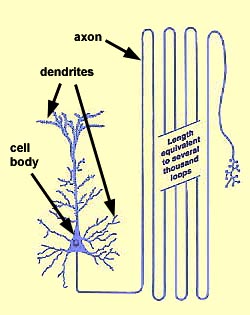
No other tissue in the body is characterized by cells whose
cytoplasmic processes reach out for vast distances away from the cells' nuclei.
This reality puts a special burden on the student
as well as the researcher. You cannot simply look at a slide or
micrograph, not even an electron micrograph, and truly "see" the most interesting
features of the nerve cell.
Nerve cell processes are quite thin, often less than
a micron (1µm) in diameter. However, the length of axons
and dendrites is wondrously great, far greater than ordinary cellular dimensions.
Dendrites may extend several millimeters away from the cell body, into a volume
the size of a pea. Axon length commonly extends for several centimeters
and may exceed a meter for many sensory and motor axons.
As a simple consequence of this cellular geometry, the
cell body of a neuron may comprise less than one percent of the cell's total
volume. From this, you may correctly infer that the bulk of nervous
tissue consists of nerve cell axons and dendrites rather than nerve cell bodies.
The study of neuroanatomy consists largely of understanding the routes
travelled by nerve cell axons.
Unfortunately, the organization of neural processes, most particularly the
full length of axons and dendrites and the synaptic interactions between them,
can seldom be visualized directly.
In most other tissues of the body, what you can see in
the microscope is directly informative. Consider skin,
where a routine section of epidermis reveals almost everything interesting
about the size, shape and growth sequence of epidermal cells. Electron
microscopy of similar specimens simply adds more finely resolved detail.
But making any sense at all of nervous tissue requires that you "see"
with concepts acquired over decades of research using many special techniques.
Historical note: One of the first challenges for
early-modern neuroanatomy consisted of mapping long-distance connections between
regions. Since it was (and remains) utterly impossible to trace individual axons
visually over their entire length, other techniques of "experimental neurology" were
needed.
One of the principal methods for nervous system mapping depended on cellular responses
to injury following lesion of a region in the nervous system of an experimental
animal.
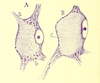
One of these responses is "retrograde degeneration," also known as the
"axon
reaction" (described in some detail by Franz Nissl in 1894), whereby damage to an axon leads
(after a suitable time interval) to alterations in the associated cell body.
By painstakingly searching throughout the brain for cell
bodies displaying the axon reaction following such a lesion, researchers could discover
(at cost to many experimental animals) the source(s) for axons passing into or through
the lesioned area.
Similarly, signs of "anterograde degeneration" or "Wallerian
degeneration" (named after
Augustus Waller, b. 1816), whereby distal portions of
a damaged nerve fiber degenerate (again after a suitable time interval) could be used
to find the destination(s) of axons originating in or passing through the lesioned
area (again, at cost to many experimental animals).
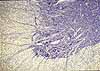 When
you examine microscope slides or micrographs of nervous tissue, patterns of
functional connection cannot usually be seen. Nevertheless, what
you can observe should be interpreted in terms of neuronal functions and connectivity,
including unseen axons, dendrites and synapses as well as associated supporting
cells.
When
you examine microscope slides or micrographs of nervous tissue, patterns of
functional connection cannot usually be seen. Nevertheless, what
you can observe should be interpreted in terms of neuronal functions and connectivity,
including unseen axons, dendrites and synapses as well as associated supporting
cells.
Thus your job for comprehending nervous tissue is not just to look-and-learn,
but to think rather deeply, to fit many different views and facts together.
Most of the listed vocabulary terms for neuronal and glial structures
are well defined in standard textbooks. You just have to make sense
of it all.
How to read this page. This page is much longer than most other pages at this histology website,
presumably because its author did his doctoral dissertation on nerve cells.
You might read this page straight down, from top to bottom. But it is written with hyperlinks to facilitate
browsing. You might more profitably check out each link, at least if
it suggests a question in your mind, and use your browser's "back"
arrow to return. And return repeatedly to the outline at the top
of this page to choose the topic that most closely engages your current curiosity.
TOP OF PAGE
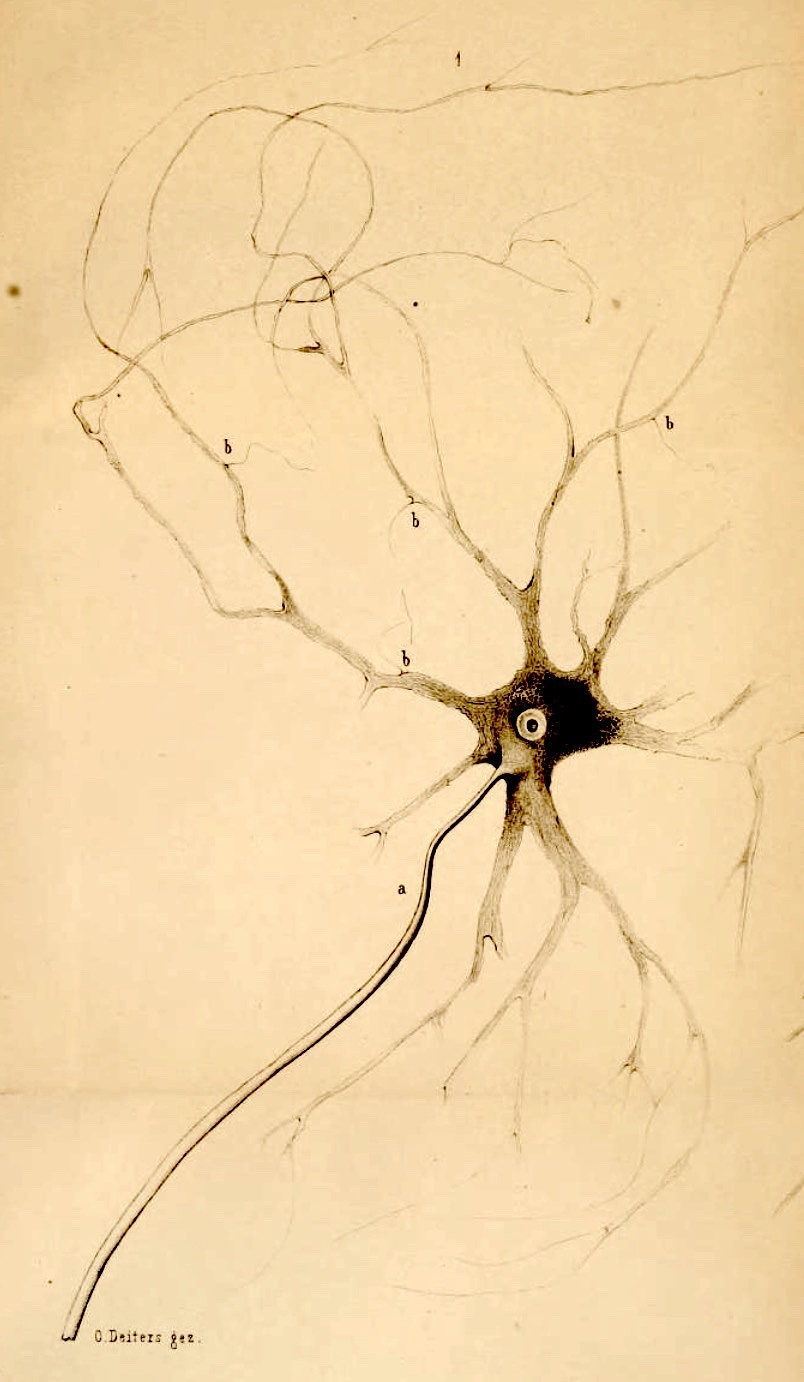 BASIC STRUCTURE OF NERVE CELLS ("NEURONS")
BASIC STRUCTURE OF NERVE CELLS ("NEURONS")
"Swiftly the brain becomes an enchanted loom, where millions of flashing
shuttles weave a dissolving pattern -- always a meaningful pattern -- though never
an abiding one" (Sherrington,
1940).
Nerve cells comprise the "enchanted
loom" that is our brain.
Here are three absolutely wonderful facts about nerve cells.
- The first and most wonderful fact is that, working together, nerve
cells can perceive and think and dream. They are us. This is
magic of the highest sort. (See my essay Cells-R-Us
for informal discussion.)
- The second wonderful fact is that nerve cells are much like other cells.
Each is essentially a bag of water, surrounded by a fatty membrane and containing
an assortment of molecules. There seems to be nothing about individual
nerve cells that cannot be explained, at least in principle, by basic chemistry
and biology.
- The third wonderful fact is that each nerve cell has a truly magnificent
shape. Somehow, this third fact bridges the gap between the mystery
that is our mind and the chemistry that is our cells. Somewhere in
the shape of nerve cells, in the complexity of connections among billions
of such cells, and in the intricate pattern of activity that plays upon
those cells, our "self" emerges.
 Nerve cells come in extreme variety. In every region
of the brain are several different nerve cell types, each distinguished
by its own characteristic soma size, dendritic shape, source of synaptic
input, destination of axonal output, and chemistry (more below).
Occasional nerve cell types may have characters which depart from the the
typical description presented below.
Nerve cells come in extreme variety. In every region
of the brain are several different nerve cell types, each distinguished
by its own characteristic soma size, dendritic shape, source of synaptic
input, destination of axonal output, and chemistry (more below).
Occasional nerve cell types may have characters which depart from the the
typical description presented below.
Because of this immense variety of nerve cell types, there
is no "one-size-fits-all" description. So textbook descriptions
of nerve cells tend to present overwhelmingly abundant detail. Although
many details of nerve cell shape and connectivity are usually insignificant for
clinical practice, they can be quite beautiful. (Ramón y Cajal
famously referred to nerve cells as "the mysterious butterflies of the soul.") And some details are essential for understanding
research on brain function. It is also often necessary to learn some
"irrelevant" detail in order to understand the particular examples
used to demonstrate basic functional principles.
*** Most of the following generalities have exceptions.
***
Every nerve cell has three distinctive portions: a cell body, one axon,
and several dendrites.
- The cell body of a nerve cell (also called the perikaryon or soma [plural somata]) is basically a cell
nucleus surrounded by cytoplasm. (Perikaryon means "around the nucleus.")
Nerve cell bodies look more or less like other body cells, although they
do have certain distinguishing features:
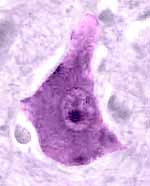
- The nucleus of a nerve cell is typically large, round and euchromatic with
a single prominent nucleolus (more below).
- Cytoplasm of a typical nerve cell body is abundantly supplied with
masses of rough endoplasmic reticulum (traditionally called
Nissl bodies, named for Franz Nissl, b. 1860),
numerous Golgi bodies (named for Camillo
Golgi, b. 1843), lots of smooth endoplasmic
reticulum, and many mitochondria. Extensive cytoskeletal elements (microtubules
and various filaments) extend from cell bodies into neural processes, where they provide
the essential framework for ongoing maintenance of axons and dendrites.
Historical note: One of the pioneers in the study of the neuronal cytoskeleton was
Sigmund Freud, during his student research into
comparative neuroanatomy of crayfish. For more on cytoskeletal research, see
The Cytoskeleton
of Nerve Cells in Historical Perspective, by E. Frixione, IBRO History of Neuroscience, 2006. This essay also
describes the role played by the neuronal cytoskeleton in development of the neuron doctrine, including significant contributions
by Ramón y Cajal
- A typical nerve cell body contains only a tiny fraction of the total cell volume;
the rest is contained in the axon and dendrites.
- The spaces between nerve cell bodies is filled with a feltwork of axonal and dendritic processes,
called neuropil, as well as glial
cells and their processes.
Extending out from each nerve cell body are long cytoplasmic processes: one axon and
several dendrites. (These processes usually cannot be distinguished
in routine histological preparation.)
- The axon is a process which is specialized for conducting signals over long distances
from one nerve cell to another.
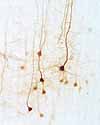 Each
nerve cell has one and only one axon, although that axon may branch extensively.
Each
nerve cell has one and only one axon, although that axon may branch extensively.
- Typical axons have relatively few branches, except near their terminal ends.
- The terminal branches of an axon make synaptic contacts onto
other nerve cells (or with peripheral effectors, i.e., muscles and glands).
- The diameter of an axon is uniform along most of its entire length.
[Recent research (Science,
05 Dec 2024, 386:1084-1085) has suggested that unmyelinated axons might, under some conditions, assume a "string of pearls" shape; the evidence
has met with skepticism as possibly representing a preparation artefact.]
- Nerve signals travel along axons away from the cell body and
toward synapses at the axonal terminal.
- On a cellular scale, the dimensions of an axon may be enormous: axonal length may exceed ordinary cellular dimensions by several orders of magnitude .
- Many axons reach a length of several centimeters. Axons in peripheral nerves can be over a meter long.
(This is in contrast to dimensions on the order of 5 to 50 micrometers for most non-neural cells.)
- The volume of an axon may be hundreds of times greater than the volume of an ordinary cell.
- The membrane surface area of an axon may be hundreds of times greater than that of an ordinary cell.
- Maintaining such vast material requires impressive metabolic machinery in the cell body as well as
efficient mechanisms for axoplasmic transport up and down the axon.
Clinical note: Certain viruses (such as the rabies virus or Herpes zoster) coopt axonal transport
mechanisms to migrate from the periphery into neuron cell bodies. Shingles results when herpes is transported from its "hiding place" in
dorsal root ganglion cells back out into the periphery.
- Damaged axons undergo "Wallerian degeneration" (named after Augustus
Waller, b. 1916), eventually dying and disappearing distal to the site of injury.
Clinical notes: If a peripheral nerve is severed or crushed, the proximal portion of each
axon, which is still connected to its cell body, can regrow distally from the site of injury. The "growth cone,"
leading this outgrowth, follows rows of Schwann cells -- provided that the damaged parts of the
nerve remain well aligned.
Following traumatic amputation, surgical repair calls for reattachment of the cut or torn ends of the epineurium to help assure
alignment for axon regrowth. Restoration of function requires axon regrowth -- a slow process of about an inch per month -- which depends on the rate of axoplasmic
transport of material from the cell body. (See, e.g., A. Höke, 2011, J. Clin. Invest. 121(11): 4231-4234.)
-
Dendrites are processes which are specialized for receiving and integrating
signals from other nerve cells. ("Integrating" is the
jargon term for what dendrites do when they combine synaptic input from several sources.)
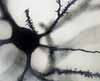 A
nerve cell typically has several dendrites, each with numerous branches.
(The word "dendrite" derives from a word meaning "tree.")
The diameter of dendrites typically decreases away from the cell body,
so that dendrites taper gradually to fine twigs.
A
nerve cell typically has several dendrites, each with numerous branches.
(The word "dendrite" derives from a word meaning "tree.")
The diameter of dendrites typically decreases away from the cell body,
so that dendrites taper gradually to fine twigs.- Dendrites typically receive synaptic contacts from axons of
many other nerve cells. Synapses often occur on tiny dendritic
spines.
- Synapses onto a single nerve cell's dendritic branches may number in the thousands, or even hundreds of thousands.
- Dendritic nerve signals, called synaptic potentials, arise
at synapses.
- Synaptic potentials are conducted passively along dendrites toward the cell body.
- Synaptic potentials fade (i.e., decrease in amplitude) with distance from their originating synapse.
- Dendrites are not myelinated, although some large dendrites may support action potentials.
- Size and shape of dendritic spines influence synaptic strength.
Plasticity of dendritic spine morphology is implicated in memory.
- Synapses are points of contact between nerve cells (usually between
axon terminals and dendrites), where signals are transmitted from one cell
to another. (Synapses can seldom be resolved without electron microscopy.)
Historical note: "Giant synapses," such as the "calyces of Held" in the brainstem
auditory pathway, are an exception. These were first reported by Hans Held
in 1893 and subsequently described in detail by Ramón y Cajal using light
microscopy. The "calyx of Held" has since become a model system for studying
synaptic function in mammalian nervous systems.
- Neurotransmission is usually chemical, based on small molecules called
neurotransmitters, secreted by one cell and binding to another.
- Neurotransmitter binding typically causes changes in membrane ion conductance,
thereby locally altering dendritic membrane potential.
- Such local synaptic potentials spread passively, to be integrated with other synaptic
potentials, typically at the axon hillock.
- (Neurotransmission can also be electrical, with
ions passing directly from one cell into another via gap junctions.)
- Each synapse has a presynaptic side: an axon terminal,
from which a neurotransmitter is released.
- Neurotransmitter is usually stored in synaptic vesicles
within the presynaptic terminal.
- Neurotransmitter is released in response to changes in membrane
potential associated with arrival of action potentials.
- Each synapse has a postsynaptic side: a dendrite or a nerve
cell body, where the membrane is specialized to respond to the binding
of neurotransmitter molecules, e.g. by altering membrane ion conductance.
- Size and shape of dendritic spines influence synaptic strength.
Plasticity of dendritic spine morphology is implicated in memory.
- Neuromodulation involves transmitters with more diffuse function (i.e., less localized to specific synapses,
less-clearly associated with discrete histological features). [More at Wikipedia.]
*** Most of the preceding generalities have exceptions.
***
TOP OF PAGE
BASIC ELECTRICAL FUNCTION of nerve cell membranes
Note: Most of this entire histology website focuses on cell and tissue structure (i.e., features which can be observed with a
microscope). My presumption in creating this website has been that most details of cell function will be covered by courses in physiology
and/or cell biology. However, because details of nerve cell shape are so closely associated with the electrical behavior of nerve cell membranes,
this section offers an outline sketch (without illustrations) of how nerve cells manipulate localized changes in cell membrane potentials in order to
carry out their functions of processing and transmitting information. (Readers not yet familiar with membrane electrochemistry are encouraged to
read this section from top to bottom at least twice, to see how the various ideas fit together.)
Skip this section (next section is myelin).
Caveat: This section is extremely elementary and highly simplified. For an alternative (and illustrated)
presentation, see links immediately below to Chapters 6 and 7 in Principles of Neural Science, 5th ed., by Kandel
et al., 2013:
Core ideas:
- Nerve cells integrate and transmit information by manipulating local changes in membrane potential.
- Local changes in membrane potential are regulated by ion channels in nerve cell membranes.
- The conductance of specific ion channels may change in response to chemical and electrical signals.
- Synaptic potentials result from neurotransmitters acting on specific ion channels in postsynaptic membranes.
- Action potentials result from stimulation of voltage-dependent sodium ion (Na+) channels in axonal membranes.
- Voltage-dependent Na+ channels characterize axons (not most dendrites), generally beginning with the axon hillock.
- Along myelinated axons, voltage-dependent Na+ channels are restricted to nodes of Ranvier.
- Voltage-dependent Na+ channels also characterize muscle fibers.
- Clinical relevance: Many drugs and toxins can modify the behavior of ion channels.
Membrane Potentials:
Ion-specific EQUILIBRIUM POTENTIALS and the NERNST EQUATION
- The concentrations of ions such as Na+ and K+ inside every cell differ from those outside.
- An ion's equilibrium potential (also called the Nernst potential
or the "reversal potential") is the voltage across a membrane which would balance the tendency of that ion to diffuse across the membrane, following
its concentration gradient.
- For any ion whose concentration differs across a membrane, that ion's equilibrium potential is given by the
Nernst equation.
- Variables in the Nernst equation are the ion's concentrations on each side of the membrane.
- For several ions whose concentrations differ across a membrane, each ion will have its own specific equilibrium potential.
- (The Nernst equation itself is based on fundamental thermodynamic principles, but the equilibrium potential for an ion can also
be determined experimentally.)
RESTING MEMBRANE POTENTIAL and the GOLDMAN EQUATION
- Every cell has an electrical potential difference (i.e., voltage) across its membrane, called the cell's resting potential.
- Resting potentials originate from differing ion concentrations between the
inside and the outside of a cell, together with variation in specific ion conductances across the membrane.
(Conductance quantifies how freely an ion can pass across the membrane.)
- At rest, the following conditions usually apply:
- Sodium ion (Na+) concentration is higher outside the cell.
- Na+ conductance is relatively low.
- Potassium ion (K+) concentration is higher inside the cell.
- K+ conductance is relatively high.
- (Calcium ions [Ca++], chloride ions [Cl-], and organic anions also contribute to membrane potentials.)
- Local changes in ion conductance can cause localized departures from the resting potential.
- For any given ion, the membrane's resting potential will generally differ from that ion's equilibrium potential.
- Therefore the resting potential is a dynamic equilibrium for multiple ions, each of which has its own particular equilibrium potential.
- Under such conditions, the resting potential entails continual movement of ions across the membrane.
- To compensate for this ongoing ion diffusion, appropriate ion concentrations inside a cell are maintained by the action of
sodium-potassium pumps, with ATP providing energy to move ions against their concentration gradients.
- The resting potential for any cell membrane is given by the Goldman equation.
- Variables in the Goldman equation include not only each ion's concentration on each side of the membrane but also each ion's
conductance across the membrane (i.e., how readily the ion can pass through the membrane via ion channels).
- (Like the Nernst equation, the Goldman equation is based on fundamental thermodynamic principles.)
- A normal cellular resting potential is dominated by K+ conductance, which at rest is substantially greater than Na+ conductance.
- Therefore, a cell's resting potential generally lies close to the K+ equilibrium potential.
- Any ion not at equilibrium at the resting potential will diffuse down its electro-chemical gradient.
- Therefore, the resting potential would decay to a stable state (where all ions have the same equilibrium potential) unless
ion concentrations were maintained by sodium-potassium pumps
which counteract diffusion while consuming energy.
Local changes in ion conductance can cause localized departures from the resting potential.
ION CHANNELS and ion conductance
- Ion movement across a membrane occurs via dedicated, ion-specific ion channels.
- Ion channels consist of trans-membrane protein complexes.
- Ion movement through ion channels follows that ion's electro-chemical gradient.
- The conductance for an ion is determined both by how freely the ion can pass through an individual channel and by the number of channels.
- The conductance at particular membrane locations may vary in response to chemical or electrical stimuli.
- Various ion channels in postsynaptic membranes respond to neurotransmitters by altering ion conductance to produce excitatory or inhibitory
synaptic potentials.
- Voltage-dependent sodium channels in axonal membranes respond to depolarizing membrane potential change by initiating action potentials.
- When "open," an ion channel allows relatively free passage.
- When "closed," an ion channel may still be "leaky," (i.e., always allowing some movement).
- Na+ and K+ each have their own dedicated channels.
- Internal Na+ and K+ concentrations are maintained by active (energy-requiring)
sodium-potassium pumps.
- Clinical relevance: Ion channels are targets not only for neurotransmitters but also for many drugs and toxins, which can alter their properties.
SYNAPTIC POTENTIALS
- Synaptic potentials are local departures from resting potential, initiated at sites of synaptic contact between nerve cells.
- Arrival of an action potential at an axon terminal triggers release of neurotransmitter.
- Neurotransmitter diffuses across the synaptic cleft and binds to transmitter receptors associated with specific ion channels in the postsynaptic membrane.
- Transmitter binding initiates processes which alter ion conductance through the associated ion channels.
- Altered ion conductance at a postsynaptic membrane causes a localized change in membrane potential at that site.
- Local postsynaptic membrane shifts may be "excitatory" (depolarizing the membrane away from its resting state) or "inhibitory" (resisting depolarization).
- Localized postsynaptic membrane potential changes cause currents which flow away from the postsynaptic site, with the result that the postsynaptic potential
spreads passively from the postsynaptic site, decreasing in amplitude over distance.
- Synaptic potentials are variable in magnitude, depending on variation in any of the factors which produce them.
- Spreading synaptic potentials from multiple synaptic sites interact with one another.
- At any given site on a dendrite or cell body, interacting synaptic potentials shift the membrane potential away from its resting state.
- If interacting synaptic potentials exceed a threshhold at an "action potential initiation site"
(a site which contains voltage-dependent sodium channels, generally located
at or near an axon hillock), an action potential will be initiated.
ACTION POTENTIALS and the Hodgkin-Huxley equations.
- Action potentials are brief, actively-propagating, all-or-nothing waves of depolarizing membrane potential.
Action potentials travel without decrement away from the site of their initiation.
- The ability to sustain action potentials is characteristic of axons and striated muscle fibers.
- The measured changes during an action potential are:
- A sudden and substantial increase in Na+ conductance, such that Na+ conductance becomes
greater than K+ conductance.
- A consequent rapid depolarizing shift in membrane potential, toward the Na+ equilibrium potential.
- A subsequent increase in K+ conductance, such that K+ is again greater than Na+.
- A consequent rapid repolarization of the membrane, as the membrane potential again approaches the K+ equilibrium potential.
- A restoration of normal, resting conductances for Na+ and K+, and hence of the normal resting potential.
- Thus an action potential consists of a rapid local depolarization of the axon membrane, followed almost immediately by a rapid repolarization,
and further followed by a brief "refractory period" during which that particular site will not generate another action potential.
- The molecules which respond initially are called "voltage-dependent sodium channels," or "voltage-gated sodium channels."
- An action potential is triggered by a depolarizing change in membrane potential which exceeds a threshhold for activating voltage-dependent sodium channels.
- Normally, action potentials are initiated at the most proximal site which contains voltage-dependent sodium channels, usually
near an axon's origin at the cell body, a site which is sometimes visibly differentiated as an "axon hillock."
- At such a site, the initiating potential change is generally the summation of many depolarizing synaptic potentials.
- Once an action potential is initiated, the spread of depolarizing potential away from the initiation site triggers a similar
response from adjacent voltage-dependent sodium channels.
- In that way, an action potential actively propagates itself away from its initiation site.
- The recovery time to restore the resting potential at a site that has just experienced an action potential prevents the action
potential from propagating "backwards" in the direction from which it had originated.
- Along myelinated axons, voltage-dependent Na+ channels are restricted to nodes of Ranvier.
- The presence of myelin increases the resistance and decreases capacitance of the axonal membrane, thereby allowing
action potentials to "jump" from one node of Ranvier to the next. This phenomenon, known as
saltatory conduction, greatly increases the speed of action potential propagation.
- (The changes in ion conductance, and hence in membrane potential, during an action potential are described quantitatively by
the Hodgkin-Huxley equations;
these equations comprise an empirical model, based on experimental measurements taken during action potentials.)
Reiterated caveat: The information in the section above is extremely elementary and highly simplified. For an alternative, illustrated
presentation, see links below to Chapters 6 and 7 in Principles of Neural Science, 5th ed., by Kandel
et al., 2013:
TOP OF PAGE
MYELIN

Myelin is a fatty covering which envelops many axons.
Myelin enables saltatory conduction, permitting action
potentials to be propagated at a much greater velocity than is possible along unmyelinated axons.
- Axons with myelin are called myelinated axons.
- Most myelinated axons are fairly large, ranging from 1µm up
to 10µm in diameter (not counting the myelin). To put this size in perspective, the diameter
of a large axon may be greater than that of a capillary.
- Axons without myelin are called, logically enough, unmyelinated
axons.
- Unmyelinated axons are usually quite small, less than 1µm in
diameter.
Myelin is formed by support cells (Schwann cells in
the peripheral nerve system, oligodendroglia in the CNS)
wrapping around the axons. Myelin is not part of, nor produced by, the
nerve cell whose axon it envelops.
In peripheral nerves, myelin consists of Schwann cell membrane wrapped
around and around an axon. Most of the Schwann cell cytoplasm lies
alongside the myelin wrapping. (See oligodendroglia for
myelination of CNS axons.)
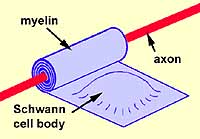 To
visualize myelin formation:
To
visualize myelin formation:
- Imagine that a Schwann cell
is a pillow with the pillowcase representing Schwann cell membrane and
the pillow's stuffing representing Schwann cell nucleus and cytoplasm.
- Next imagine a broomstick (representing the axon)
lying across one end of the pillow.
- Now roll the broomstick up in the pillow, wrapping
the pillowcase tightly around and around the broomstick while squeezing
the pillow's stuffing into one end.
- The tight wrappings of pillowcase now represent the
myelin, while the remaining pillow with stuffing represents the Schwann
cell body with nucleus and cytoplasm.
|
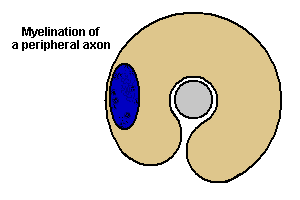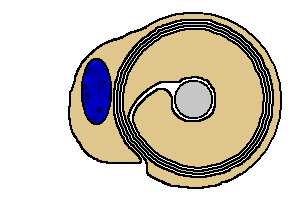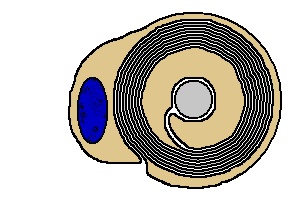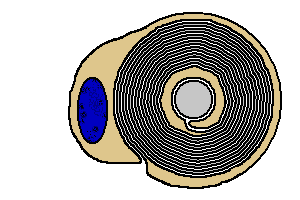
Myelination of a peripheral axon
Animation from Blue
Histology, copyright
Lutz Slomianka 1998-2004
(The image should be animated, if
you watch patiently.)
 A
Schwann cell is illustrated with brown
cytoplasm. A
Schwann cell is illustrated with brown
cytoplasm.
The blue oval is the Schwann
cell's nucleus.
Observe that as the growing Schwann cell spirals
inward around the axon, it wraps its membrane into layers of myelin.
|
The myelin of one Schwann cell wraps about one to two millimeters along an
axon. To myelinate the entire length of the axon, many of these Schwann
cell wrappings line up end-to-end along the axon.
 The points between segments of myelin are called nodes of Ranvier
(named after Louis Ranvier, b. 1835).
The stretch of axon between nodes is called an internode.
The points between segments of myelin are called nodes of Ranvier
(named after Louis Ranvier, b. 1835).
The stretch of axon between nodes is called an internode.
DETAIL: Cytoplasm around a Schwann cell's nucleus is connected -- by narrow channels which spiral inward beside the nodes of Ranvier at either end of an internode -- to the cytoplasm alongside the axon. Similar channels within the internode are called Schmidt-Lanterman clefts (commemorating H.D. Schmidt, b. 1823, and A.J. Lanterman, b. 1845).
NOTE:Many details of myelin cannot be well-appreciated by light microscopy.
For electron micrographs of myelin in peripheral nerves, see the online
Electron
Microscopic Atlas of cells, tissues, and organs (the text is in German, but most
figure labels can be deciphered fairly easily).
TOP OF PAGE
SALTATORY CONDUCTION
The spacing of nodes of Ranvier is critical for propagation of action potentials. Along
myelinated axons, action potentials are regenerated only at the nodes. Myelin
provides insulation -- and, more importantly, decreased capacitance -- so that
the ionic currents at one node can flow efficiently (and quickly) to the
next node. This is called saltatory conduction (saltation =
jump). In contrast, action potentials propagating along unmyelinated
axons are regenerated at each point along the way, a much slower process.
[A hydrodynamic metaphor for saltatory
conduction may offer a somewhat intuitive explanation.]
Clinical note, multiple sclerosis: Because myelinated axons have voltage-dependent
sodium channels only at nodes of Ranvier, even localized sites of demyelination, such as those which occur in
multiple sclerosis, can effectively prevent the propagation of action potentials across the lesion.
Clinical note, local anesthesia: As long as myelin is intact, local currents generated by
an action potential at one node of Ranvier are generally sufficient to depolarize axonal
membrane two or three nodes away. Local anesthesics, which block action potentials but do
not prevent current flow, must therefore be distributed across several nodes (several millimeters)
in order to produce effective anesthesia.
TOP OF PAGE
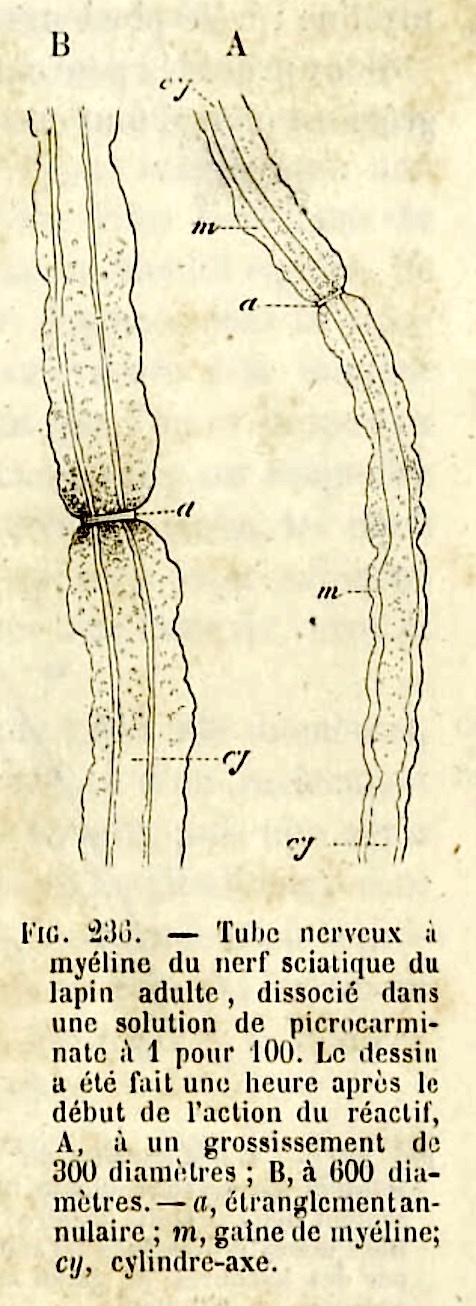
 RECOGNIZING NERVE CELLS in histological preparations.
RECOGNIZING NERVE CELLS in histological preparations.
Although axons reach into all parts of the body, the vast majority of nerve
cell bodies occur in the central nervous system (brain and spinal cord),
in those regions described as gray matter. Relatively
few nerve cell bodies occur peripherally, in the ganglia
(small clusters of nerve cells) of sympathetic and parasympathetic nervous
systems.

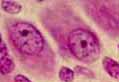
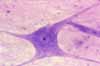
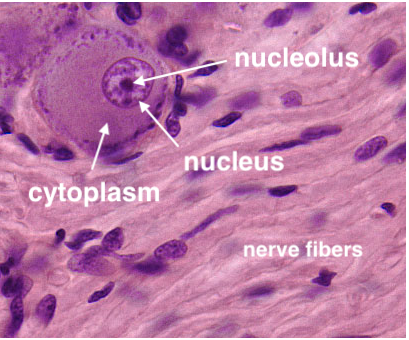 Wherever they occur, most nerve cell bodies have a distinctive appearance.
Wherever they occur, most nerve cell bodies have a distinctive appearance.
- Nuclei of nerve cells are large, round and
euchromatic with a single prominent nucleolus. Because of this distinctive
nuclear appearance, neurons are sometimes described as having "owl-eye"
nuclei or "fried-egg" nuclei.
- Cytoplasm of all but the smallest nerve cell bodies is substantial
and conspicuously basophilic, containing characteristic basophilic masses
of rough endoplasmic reticulum that are traditionally called Nissl
bodies (commemorating Franz Nissl, b. 1860).
These features of nerve cell bodies are related to the heavy metabolic
demands imposed by maintaining extensive cytoplasmic processes (i.e., axons and dendrites).
They are exaggerated (i.e., bigger nuclei, more cytoplasm) in those nerve cells which have the longest,
largest diameter axons.
Nerve cells with the most extremely long, large diameter
axons -- such as pyramidal cells of motor cortex
and motor neurons of spinal cord -- are often
illustrated as "typical" neurons simply because they are big and
hence especially easy to visualize. Cerebellar Purkinje
cells comprise another "popular" type of nerve cell, also
large but with a huge dendritic tree rather than an especially long axon.
TOP OF PAGE

Special stains, like the silver-based Golgi
stain, can reveal entire
neurons or glial cells (at least as much as fits within the thickness of a
single section) by impregnating them with opaque silver salts. But this technique
yields elegant results only by suppressing any staining of most neighboring
cells, so neurons appear in splendid isolation when their essence is one of
complex interaction. Similarly, electron microscopy
can display elegant synapses, but the narrow view offers few clues about the
cells to which the pre- and post-synaptic profiles belong.
Historical note: The Golgi stain was discovered by
Camillo Golgi and was famously exploited by Santiago
Ramón y Cajal to develop the Neuron Doctrine.
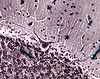 Sections of central nervous tissue routinely show neuron cell bodies surrounded by
a finely-textured fibrous material often called neuropil (which should
not be confused with connective tissue).
This feltwork consists of axons and dendrites (and glial processes),
with all the comings and goings that these processes entail. Individual
axons and dendrites can be distinguished only in fortuitous sections, and
then only for a short length. The so-called "molecular" layers
of cerebral and cerebellar cortex consist of neuropil containing relatively
few cell bodies (most of the cell bodies lie in deeper layers).
Sections of central nervous tissue routinely show neuron cell bodies surrounded by
a finely-textured fibrous material often called neuropil (which should
not be confused with connective tissue).
This feltwork consists of axons and dendrites (and glial processes),
with all the comings and goings that these processes entail. Individual
axons and dendrites can be distinguished only in fortuitous sections, and
then only for a short length. The so-called "molecular" layers
of cerebral and cerebellar cortex consist of neuropil containing relatively
few cell bodies (most of the cell bodies lie in deeper layers).
Note that a common artefact, resulting from tissue
shrinkage, is for a clear "halo" to appear around cell
bodies and blood vessels. Although the presence of such halos can
be misleading (there is no such space in intact, living nervous tissue),
this consistent artefact serves to highlight or emphasize the locations
for these structures.
TOP OF PAGE
SUPPORT CELLS of nervous tissue.
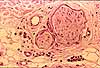 Schwann cells are support cells in peripheral nerves (named after
Theodor Schwann, b. 1810).
Schwann cells are support cells in peripheral nerves (named after
Theodor Schwann, b. 1810).
- Schwann cells form the myelin around myelinated
peripheral axons.
- Schwann cells also envelop unmyelinated axons, but without the
close, dense membrane wrapping which characterizes myelin.
Clinical note: When a peripheral nerve is crushed or severed, the distal portion
of each axon undergoes Wallerian degeneration. However, Schwann cells distal to
the injury may remain intact (although their myelin does degenerate); the linear arrangement
of Schwann cells can then serve to guide axon regrowth during recovery.
Many of the small, heterochromatic nuclei that can be seen within peripheral
nerves belong to Schwann cells. Some of the remaining nuclei
belong to fibroblasts of
the endoneurium, perineurium, and epineurium
(i.e., connective
tissue) that give tensile strength to the nerve. Perineurium also
contains squamous perineural cells (perineural epithelium) which form
a continuous layer that isolates the axons within from surrounding connective
tissue.
Fibroblast nuclei tend to be smaller and more densely heterochromatic
than Schwann cell nuclei, but in most ordinary preparations that include
peripheral nerves, it is impractical to distinguish these nuclei.
Note that none of the nuclei visible in peripheral nerves belong to nerve
cells. Peripheral nerves do NOT contain nerve cell bodies, only axons
of nerve cells whose cell bodies lie elsewhere.
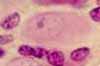
Support cells in peripheral ganglia are sometimes called satellite cells.
Schwann cells can form tumors called schwannomas.
TOP OF PAGE
Glial cells -- Support cells of the CENTRAL NERVOUS SYSTEM
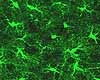
 The
most numerous cells within the central nervous system are glial cells.
The name "glia" means "glue" (filling the interstices
of nervous tissue), reflecting old but enduring ignorance of their function
(and the inadequacy of classical histology to offer much insight). The
small nuclei of glial cells may be readily observed in any section of central
nervous tissue. Unfortunately, like neurons, these cells are difficult
to visualize satisfactorily.
The
most numerous cells within the central nervous system are glial cells.
The name "glia" means "glue" (filling the interstices
of nervous tissue), reflecting old but enduring ignorance of their function
(and the inadequacy of classical histology to offer much insight). The
small nuclei of glial cells may be readily observed in any section of central
nervous tissue. Unfortunately, like neurons, these cells are difficult
to visualize satisfactorily.
Although glial cells vastly outnumber nerve cells (approx.
10:1, glia:neurons), nerve cells are so large, including the total volume
of all their dendrites and axons, that most of the cellular volume of the
brain consists of nerve cells.
Ignorance of glial function is beginning to dissipate. For a 2008 review, see:
Barres,
BA (2008) The mystery and magic of glia: a perspective on their roles
in health and disease. Neuron. 60 (3): 430-40 [PubMedID: 18995817]:
This perspective reviews "recent evidence
that glial cells are critical participants in every major aspect of brain
development, function, and disease. Far more active than once thought,
glial cells powerfully control synapse formation, function, and blood flow.
They secrete many substances whose roles are not understood, and they
are central players in CNS injury and disease. I argue that until
the roles of nonneuronal cells are more fully understood and considered,
neurobiology as a whole will progress only slowly. ... And please don't forget the glia! Quite
possibly the most important roles of glia have yet to be imagined" [emphasis added].
Recent research: In 2025, the journal Science published a report that
"astrocytes are indispensible for neuromodulatory signalling across diverse neural circuits,
behavioral contexts, and species" (G. Eroglu, "Astrocytes, hidden puppet masters of the brain,"
Science 388:705-6).
The two most common types of glia, oligodendroglia
and astroglia, both have extensive cytoplasmic
processes and are intimately involved in the function of nervous tissue. A
third glial type, microglia, function similarly
to macrophages.
 In
most of our reference slides, both in the spinal smear and in sections of
brain and spinal cord, glial cells are revealed only by their nuclei, with
little indication of cytoplasmic shape. The characteristic processes of
glia can show up nicely in some of the Golgi-stained sections in your reference
collection (variously cerebellum or cerebral cortex). However, even with electron
microscopy, it is difficult to trace CNS myelin to the arms of the oligodendroglia
from which it forms.
In
most of our reference slides, both in the spinal smear and in sections of
brain and spinal cord, glial cells are revealed only by their nuclei, with
little indication of cytoplasmic shape. The characteristic processes of
glia can show up nicely in some of the Golgi-stained sections in your reference
collection (variously cerebellum or cerebral cortex). However, even with electron
microscopy, it is difficult to trace CNS myelin to the arms of the oligodendroglia
from which it forms.
Separately distinguishing among astroglia, oligodendroglia
and microglia is a skill for specialists (i.e., pathologists),
but with practice their nuclei can be recognized by relative size and texture,
with astrocyte nuclei being somewhat larger and paler than the others.
TOP OF PAGE
Oligodendroglia (also called "oligodendrocytes"
or just "oligos") typically have relatively few processes (hence
their name; oligo = few), with each process ending in a sheet of myelin
which wraps around a segment of an axon.
Function of oligodendroglia:
Oligodendrocytes form myelin in the CNS and hence are responsible
for normal propagation of action potentials.
Patchy loss of CNS myelin, as in multiple sclerosis, can cause a variety of neurological problems.
Myelin formation by oligodendroglia
is slightly different than that by Schwann cells,
each of which wraps myelin around a single axon. Each of the several
glial cell processes extends to and then myelinates a segment of one axon.
If the myelin of one oligodendrocyte process were unrolled, the process
would be shaped rather like a wide-bladed shovel (the thin shovel blade
would represent the membrane that rolls around the axon to form myelin, and
the shovel handle would represent the process which extends back to the
glial cell body). Each oligodendroglial cell has several such "shovels,"
forming myelin around several axons.
Recent evidence from mouse, based on gene transcription profiles, indicates that oligos form several
populations. For example, "One population was responsive to motor learning, and another, with a different
transcriptome, traveled along blood vessels" (Science, 10 June 2016, 352:1288-1290,
DOI: 10.1126/science.352.6291.1288-n).
TOP OF PAGE
Astroglia or astrocytes
extend branching cytoplasmic processes in all directions (yielding the star-like
shape suggested by their name; astro = star). Foot-processes
of astrocytes line every surface where central nervous tissue contacts other
body tissues, not only the obvious outer surface immediately underlying the
pia mater (where they form the glia limitans) but also along every
blood vessel and capillary which penetrates into the brain and spinal cord.
Other astrocyte foot processes approach nerve cells at any sites where
the nerve cell membrane is not otherwise occupied by synapses or by oligodendroglia.
Functions of astroglia:
There has been growing awareness this century that astrocytes play several critical roles.
Astrocyte functions and pathologies include all of the following [as of 2003, from
Ransom, et al., "New roles for astrocytes (stars at last)," Trends in Neuroscience, 26:520-522, 2003;
doi:10.1016/j.tins.2003.08.006].
- Homeostasis, regulating concentrations of K,+
extracellular pH, glutamate and water.
- Maintaining integrity of the blood-brain barrier.
- Modulation of excitatory and inhibitory synapses.
- Neuronal pathfinding during development and regeneration.
- Glioma formation.
- Cytotoxic brain edema.
- Modulation of stroke outcome.
- Hepatic encephalopathy.
- Trophic modulation of neural repair and axon regrowth
following injury.
Additional astrocyte functions:
Interactions between central glial cells [astrocytes and microglia] and neurons in the pain circuitry
contribute to the pathogenesis of chronic pain [see e.g.
Neurotherapeutics
(2020) 17:846-860; also see 2021
news article in The New York Times].
Activity of individual astrocytes can correspond
closely with that of associated neurons, and can also modulate local blood
flow (Schummers, et al., Tuned responses of astrocytes and
their influence on hemodynamic signals in the visual cortex, Science
320:1638-1643, 2008; doi:10.1126/science.1156120).
Research has also indicated that astroglia participate in the "glymphatic
system" which allows recirculation of CSF and brain interstitial
fluid along paravascular channels, a system implicated in sleep
(Science
news article, 2013).
Serotonin induces changes in gene expression by astrocytes
(Science
news article, 2023).
"Astrocyte signalling pathways influence neuronal networks and behavioral responses to neuromodulators" (Science perspective, 2025).
TOP OF PAGE
Microvascular control: Local variation in blood flow through
brain capillaries may be regulated by activity of pericytes,
which in turn can respond to neural activity. [Reference:
MacVicar & Salter, Neuroscience:
Controlled capillaries, Nature
443, 642-643 (12 October 2006) | doi:10.1038/443642a.]
The Blood Brain Barrier
"Blood-brain barrier" is the name given to a physiological
property of CNS blood vessels. In contrast to vessels in most
other parts of the body, vessels in the brain do NOT allow most molecules to pass freely between
blood and interstitial spaces of the brain. The integrity of the blood-brain
barrier is established by continuous capillary
endothelium together with the absence of endothelial
vesicular transcytosis. The only substances which cross this
barrier are those which can diffuse through endothelial plasma membranes
or those for which specific endothelial membrane channels exist.
The blood-brain barrier is a concept with considerable clinical
significance, not only because it limits the delivery of drugs to
the central nervous system but also because pathological disturbance
of the barrier can seriously impact brain function. For
more extensive information, see Blood Brain Barrier
at the University of Arizona Health Science Center; at this website,
click on ABOUT THE BLOOD BRAIN BARRIER for a drop-down menu.
|
TOP OF PAGE
Microglia are small cells, comprising about
10% of the total brain cell population, which represent the brain's immune
system (i.e., macrophage-equivalents residing within the brain). Microglia
are also implicated in the maturation, plasticity, and remodelling of synaptic
circuits (Science
333:1391, 9 September 2011, doi:10.1126/science.1212112;
J. Neurosci. 31 16064-16069, 2011 doi:
10.1523/jneurosci.4158-11.2011)
As described by Kembermann and Neumann (Microglia: the
enemy within? Science
302:1689, 5 December 2003, doi:10.1126/science.1092864),
the brain exhibits "a robust innate immune response thanks to its microglia,
which defend against invading microorganisms and clean up by engulfing the
debris of dying cells. In addition, the inflammatory mediators released
by microglia during an innate immune response strongly influence neurons
and their ability to process information." Recent in vivo observations
(Fetler and Amigorena, Brain under surveillance: the microglia patrol, Science
309:392-3, 15 July 2005, doi:10.1126/science.1114852)
show microglia as surprisingly dynamic cells, continually extending and
withdrawing fine motile cellular processes and contacting astrocytes, neurons,
and blood vessels.
Recent research indicates that microglia (in mice) are
"an ontogenically distinct population in the mononuclear phagocyte
system," originating during embryonic development (Science,
29 October 21, 2010; DOI: 10.1126/science.1194637)
"Interactions between central glial cells [astrocytes and microglia] and neurons in the pain circuitry are critical contributors to the pathogenesis
of chronic pain [Neurotherapeutics (2020) 17:846-860]. (Also see 2021
news article in The New York Times.)
TOP OF PAGE
Blood vessels in CNS
Central nervous tissue is highly vascular, so blood
vessels should be a significant feature in any histological specimen of
CNS. Large vessels generally remain on the surface of the brain
or spinal cord, so only smaller vessels penetrate into gray and white matter.
Clinical note: Understanding the course of blood vessels serving various regions of the brain is critical for understanding
the effects of stroke when flow through a vessel is compromised.
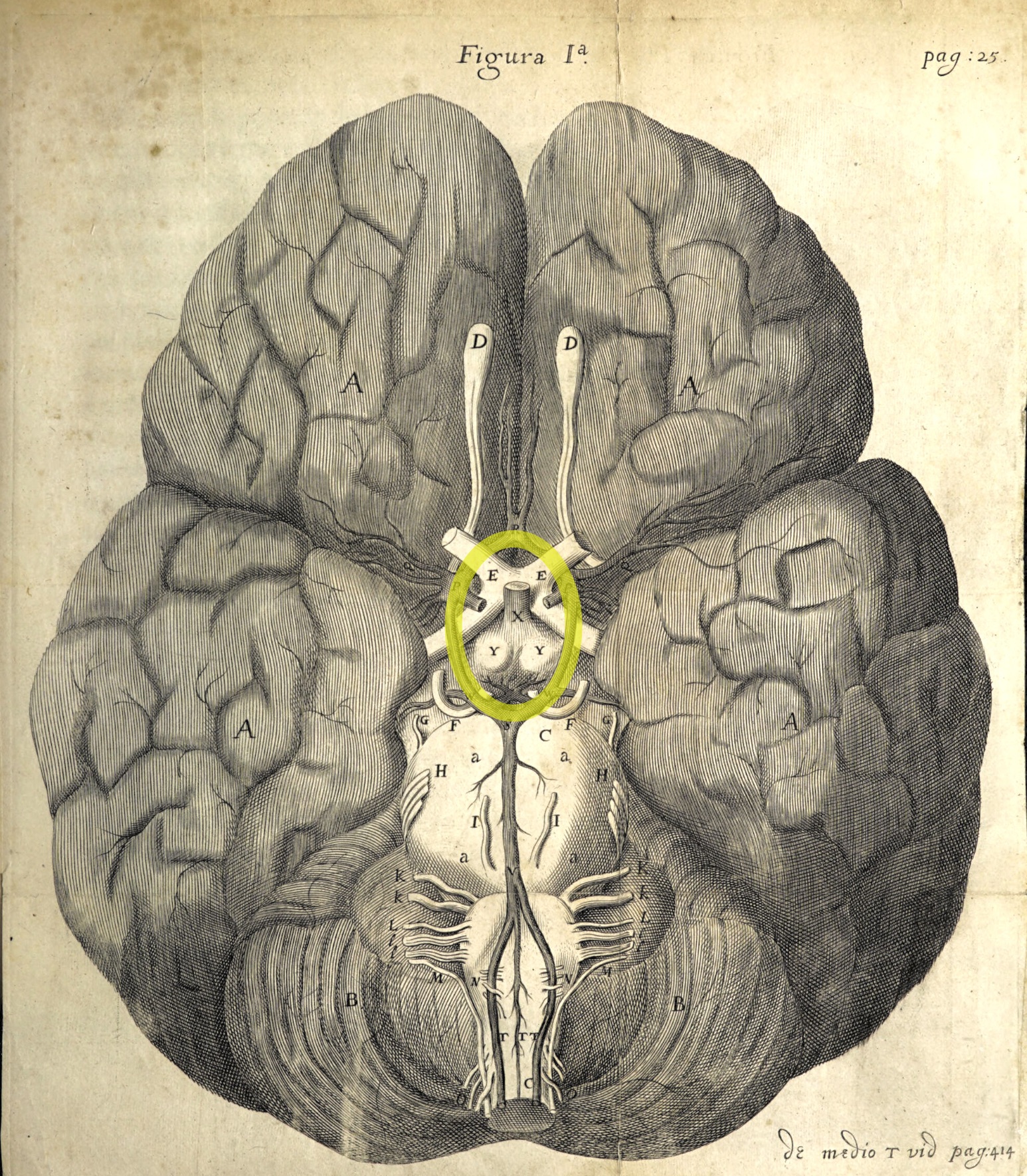
Historical note: Cerebral vasculature was first described in detail in 1664
in Thomas Willis's Cerebri anatome: cui accessit
nervorum descriptio et usus. Willis is commemorated in the circle of Willis,
a ring of interconnected arteries at the base of the cerebrum
In histological sections, small vessels may not be immediately recognizable as such. As
in other regions of the body, capillaries may be quite inconspicuous due to
small size. Even venules and arterioles may be small enough that the
layers in their walls are not clearly visible. Blood cells may be washed
out during preparation. Nevertheless, such vessels should be noticed,
since they play a crucial role in brain function and pathology. (Also
see note on microvasculature, above.)
Blood vessels are generally the largest structural elements in both neuropil and
white matter (i.e., even capillaries are larger in diameter than most CNS
axons and dendrites). The thumbnails below link to several spinal cord specimens
in which blood vessels may be observed. Blood vessels appear similar
in any region of the brain.
Note that a clear "halo" commonly appears around
blood vessels (as well as around cell bodies of neuronal and glial cell bodies).
The size of this space is an artifact of histological
preparation, resulting from tissue shrinkage when the central nervous tissue
is fixed. But it is also a reminder that vessels in the brain are surrounded by
usually-inconspicuous perivascular space, which is an extension of subarachnoid space.
TOP OF PAGE
Ependyma, choroid plexus and cerebrospinal fluid
The ventricular system of the brain is lined by a simple cuboidal epithelium called
ependyma, a remnant of the embryonic neuroectoderm which once formed
the neural tube. At certain sites -- the posterior margin of the lateral
ventricles, the midline of the 3rd ventricle, the roof of the 4th ventricle --
this ependyma lies adjacent to overlying connective tissue. Here
the ependyma is extensively wrinkled to form choroid plexus, together with blood vessels
which are caught up in the folds of ependyma.
Choroid plexus is the source for cerebrospinal fluid (CSF). CSF
is actively secreted by the ependymal cells of choroid plexus and (like
aqueous humor in the eye) accumulates at a steady rate even if drainage
points become occluded.
This is one of three sites associated with the nervous system where a special fluid is
produced by a unique tissue, with this fluid needing an outlet elsewhere to avoid buildup of
pressure. (The other two sites are the eye and the
inner ear. In the eye, aqueous
humor secreted by ciliary processes is drained through the
canal of Schlemm. In the ear, endolymph
secreted by stria vascularis is drained through the
endolymphatic sac.) In each of these sites,
an imbalance between production and drainage can cause neurological symptoms.
In composition, CSF differs considerably from blood. Although
osmolarity and sodium concentrations are similar in blood and CSF, CSF has
somewhat more chloride; less potassium, calcium, magnesium
and glucose; much less protein, and practically no white blood cells.
For specific values as wells as alterations in disease, see Kandel
et al., 4th edition, Appendix B, especially pp. 1295-1299.
Research news (2013, 2024): CSF and brain interstitial fluid are exchanged through the so-called "glymphatic
system" of paravascular channels. A 2013 report in Science
342:373 implicates this system in the function of sleep (Science
news article). A more recent (2024) Science
Insight article suggests that "Glymphatic-lymphatic brain cleansing may reveal new therapeutic strategies."
NOTE: This writer is unclear about the distinction between paravascular space and perivascular space. See,
for example, here and
here.
Research news (2023): Research continues into the immune-related relationships among choroid plexus, CSF, brain interstitial
fluid and perivascular channels. For a recent (2023) review (beginning with a short summary), see
Science
7 April 2023, p. 52.
 The layout of choroid plexus is perhaps most easily appreciated embryologically
-- click on the thumbnail for an image of embryonic choroid plexus.
The layout of choroid plexus is perhaps most easily appreciated embryologically
-- click on the thumbnail for an image of embryonic choroid plexus.
Cerebrospinal fluid accumulates not only from the action of choroid
plexus but also from the interstitial spaces of the brain. It flows,
under positive pressure developed by its active secretion, through the ventricular
system, thence out through holes in the roof of the 4th ventricle into the
subarachnoid space, finally draining through "arachnoid
villi" into the venous sinuses of the cranial cavity.
TOP OF PAGE
Meninges: dura mater, pia mater, and arachnoid
The central nervous system is enveloped by
specialized layers of connective tissue.
- The outermost layer is the dura mater (or just "dura"),
very dense fibrous connective tissue, tough and fairly impermeable.
- Immediately adjacent to the brain is the pia mater (or just "pia"),
a delicate layer of collagen and fibroblast-like cells that adheres closely
to the underlying glia limitans (the outermost layer of proper nervous tissue).
- In between dura and pia is the arachnoid, a layer of very loose
connective tissue in which cerebrospinal fluid occupies
the position of ground substance.
- The name "arachnoid" presumably refers to
the spidery, or delicately web-like, network of collagen fibers which
extend through the arachnoid layer from dura to pia.
- Perivascular extensions of the subarachnoid space, following blood vessels into
the brain, are called "Virchow-Robin space" (the name commemorates
Rudolf Virchow, b. 1821, and C.-P. Robin, b. 1821).
- Pia and arachnoid are not distinct, separate layers;
together they are sometimes called pia-arachnoid.
- The fluid-filled spaces of the arachnoid layer are
sometimes called the subarachnoid space. However,
in spite of the "sub" this space is within
the arachnoid layer.
- Where the dura envelops cerebral venous sinuses, it is
perforated by small passageways called "arachnoid villi" or
"arachnoid granulations." These are sites where cerebrospinal
fluid drains from the subarachnoid space into venous blood.
TOP OF PAGE
SOME EXAMPLES of nervous tissue.
This section offers a guide for microscope lab (i.e., for viewing slides in
your reference set). Most of these slides are unlike anything a physician is
likely to encounter in practice. What these slides do provide is an opportunity
to see for yourself certain features of nervous tissue which are more-or-less readily
accessible to microscopic viewing.
TOP OF PAGE
Spinal cord smear
 Using your reference slides, the best view of "whole" neurons is provided by the
slide labelled "nerve cells, ox spinal cord." (This is a slide of spinal
smear, not a slice but a small amount of gray matter squished
onto the slide.)
Using your reference slides, the best view of "whole" neurons is provided by the
slide labelled "nerve cells, ox spinal cord." (This is a slide of spinal
smear, not a slice but a small amount of gray matter squished
onto the slide.)
- Each spinal neuron displays classic nerve cell characteristics:
- prominent cell body,
- distinctive round euchromatic nucleus,
- single prominent nucleolus,
- cytoplasm with basophilic masses of Nissl substance (rough endoplasmic
reticulum).
The largest nerve cells in this preparation represent
spinal motor neurons, the cells whose very long
axons extend out peripheral nerves to the muscles. From the nerve cell
body extend several dendrites. These are broad at their base and contain
Nissl bodies. But dendrites decrease in diameter and basophilia with increasing distance from
the soma. The full extent of the dendritic arborization is not visible,
since the fine distal branches are obscured in the background texture of the
slide.
Each neuron also has a single axon, which can be readily identified
only if it begins on the edge of the cell body (as opposed to the top or bottom,
as viewed in the slide). The axon, unlike the dendrite, has a uniform
diameter and does not contain basophilic Nissl bodies. It begins at
the axon hillock, a specialized site on the cell body where the cytoplasm
is clear (like the axoplasm, it lacks Nissl bodies). The axon, even
more so than the dendrites, disappears into the distance and cannot be followed
to its end.
In this same preparation, smaller cells with similar features represent spinal
interneurons. Scattered throughout this preparation
are also very many cells whose nuclei are smaller than those of the neurons,
oval with clumps of heterochromatin, and whose cytoplasm is inconspicuous.
These are the glial cells. Numerous capillaries,
narrow tubular profiles wandering across the slide, may also be seen.
TOP OF PAGE
 Spinal cord section
Spinal cord section
The spinal cord consists of ascending and descending axonal
pathways (i.e., white matter) surrounding a central
core of gray matter. Use your preferred neuro text
to rehearse the functions associated with the following regions in the spinal
cord.
 The
dorsal horns are the narrower regions of gray
matter which extend to surface on the dorsal, or posterior, aspect of
the cord.
The
dorsal horns are the narrower regions of gray
matter which extend to surface on the dorsal, or posterior, aspect of
the cord.
- The ventral horns are the broader regions of gray
matter which do not extend to the surface of the cord.
- The conspicuous nerve cell bodies in the ventral
horn belong to spinal motor neurons. These
spinal motor neurons are lost
in ALS, amyotrophic lateral sclerosis.
- The relative size of the ventral
horn in any particular section depends on the level in the spinal cord where the section was taken.
The ventral horn is bulkier at levels that connect with arms or legs.
- Dorsal columns are the white-matter fiber
tracts between the dorsal horns.
- Lateral and anterior columns are the white-matter
fiber tracts which form the sides and front of the cord.
- White matter columns are thicker (containing more axons) in the upper cord, reflecting the
number of axons at each level. (For example, as one ascends the cord, more and more sensory axons
join the dorsal column.)
- The central canal is the small channel within the "bridge" of
gray matter which connects the gray matter of left and right sides.
The central canal should properly be lined by ependyma (epithelial tissue),
but this is seldom visible on our slides.
 Some sections of spinal cord may include dorsal and ventral roots containing (respectively)
sensory and motor axons.
Some sections of spinal cord may include dorsal and ventral roots containing (respectively)
sensory and motor axons.
- Sensory axons in a sensory (dorsal) root enter the cord
at the dorsal horn. Cell bodies of sensory axons occur in dorsal root
ganglia, located near the cord along the dorsal root.
- Motor axons in a motor (ventral) root originate from
motoneuron cell bodies in the ventral horn and pass through white matter
before leaving the cord.
TOP OF PAGE
Cerebral cortex
The cerebral cortex forms the surface of gyri and sulci over each entire
cerebral hemisphere. Its composition is complex (after all, it is the
seat of conscious perception and thought!), with many different types of nerve
cells. These include many local interneurons
(stellate cells and granule cells)
as well as the much larger and more conspicuous pyramidal
cells, some of whose axons enter the underlying white matter and travel
to other cortical areas or to other regions of the brain.

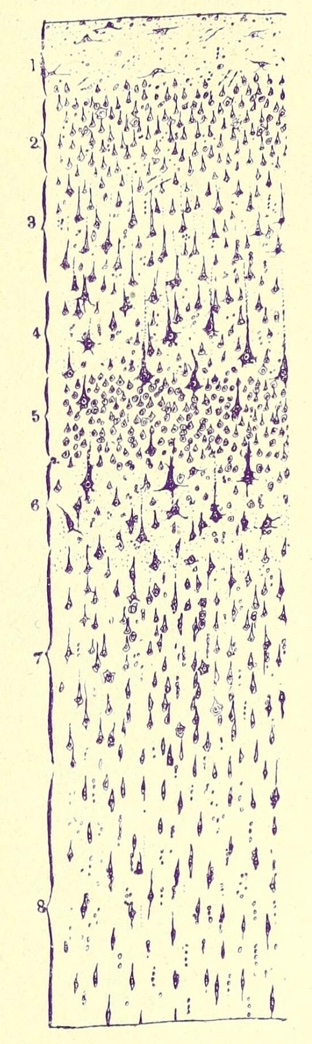
 The cerebral cortex is traditionally (but rather arbitrarily) described as having
six layers. Functional localization in the cortex correlates with noticeable differences in the
numbers and sizes of cell bodies in these several layers, now mapped as
Brodmann's areas. Although the several layers are not clearly demarcated
(they are arbitrary, after all), they can be roughly approximated by looking
for the following features.
The cerebral cortex is traditionally (but rather arbitrarily) described as having
six layers. Functional localization in the cortex correlates with noticeable differences in the
numbers and sizes of cell bodies in these several layers, now mapped as
Brodmann's areas. Although the several layers are not clearly demarcated
(they are arbitrary, after all), they can be roughly approximated by looking
for the following features.
Layer I (the "molecular
layer") is the outermost layer. This layer contains relatively
few nerve cell bodies. The odd name "molecular layer"
derives from the fine texture of this layer, due to its composition largely
of dendrites and fine axon terminals (and glia, of course). This layer might
possibly have a special role in memory
(Science
374:538, 20 Oct. 2021).
Layer II (the "outer granular layer"),
typically contains many very small cells (granule cells).
Layer III (the "outer pyramidal layer")
contains cell bodies of small pyramidal cells. Axons from these
cells typically project to the upper layers of neighboring cortical regions.
Layer IV (the "inner granular layer")
contains axonal ramifications of afferent fibers, such as sensory
axons from the thalamus. Axons from the lateral geniculate nucleus
(the visual relay of the thalamus) are so numerous that the primary visual
cortex which receives these axons (Brodmann's area 17, at the occipital
pole of each hemisphere) is sometimes called "striate cortex,"
because these axons conspicuously divide the cortex into layers that are
visible to gross inspection.
Layer V (the "inner pyramidal layer")
contains cell bodies of large pyramidal cells.
Axons from these cells typically project to more distant cortical
regions, to other parts of the brain, or to lower centers (such as spinal
motor neurons). The larger size of these pyramidal cell bodies (compared
the the smaller cells of layer III) is associated with the greater length
of their axons. (Recall that cell bodies provide most of the basic
cellular functions needed to maintain the axon, while the axonal surface
membrane and axoplasmic volume may be many times greater than the surface
and volume of the cell body.)
Layer VI (the "layer of pleiomorphic cells)
typically contains cells of assorted size and shape (hence, "pleiomorphic").
Deep to layer six is white matter containing
axons going to and from the cortex.
Historical note: Regional variations across the cortex in
the "cytoarchitecture" (detailed histological appearance) of these several cortical layers were
described over a century ago by Korbinian Brodmann (b. 1868).
Brodmann's descriptions formed the original basis for recognizing
Brodmann's areas,
now known to correspond with functional localization in the cortex.
TOP OF PAGE
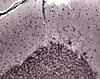
 Cerebellar cortex
Cerebellar cortex
The cortex of the cerebellum consists of three well-defined layers.
The most prominent nerve cells are Purkinje
cells, whose cell bodies all lie at the same level.
The outer molecular layer consists principally of the dendrites of Purkinje
cells and the axons of granule cells. The odd name "molecular
layer" derives from the fine texture of this layer, due to its composition
largely of dendrites and fine axon terminals. Nuclei in this layer
belong mostly to glial cells.

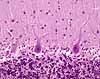
The Purkinje cell layer contains large cell bodies
of Purkinje cells, the sole output cells for the
cortex.
The inner layer, or granule cell layer,
is packed with nuclei of vastly many cerebellar granule
cells. These are among the smallest (and most numerous) neurons
in the body.
Deep to the granular cell layer is white matter containing
axons going to and from the cortex.
The
pattern of connections among various axons and dendrites in the cerebellum
is extremely elegant and regular, and has been described in extensive detail.
Any thorough neuro text (e.g., Kandel et al., 4th ed., pp. 835 ff)
should have a good account.
TOP OF PAGE

Peripheral ganglia
Both the paravertebral ganglia of the sympathetic nervous system and the
scattered ganglia of the parasympathetic nervous system consist of small clusters
of nerve cell bodies.
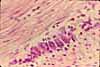
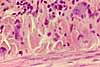
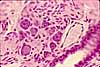
Paravertebral dorsal root ganglia contain the cell bodies for somatic sensory nerves.
Parasympathetic ganglia may turn up in sections
of various visceral organs, where they can be recognized by the classic
appearance of nerve cell bodies.
TOP OF PAGE
 Retina
Retina
Tissues of the eye are listed in a separate page.
TOP OF PAGE
Composition and appearance of PERIPHERAL NERVES
Like other portions of the nervous system, peripheral nerves are a part of
a functioning, highly organized whole. Each portion must be
understood in relation to the rest of the system.
Examples of peripheral nerves are often fairly easy to find in sections of
the skin. Larger nerves often run
in parallel with blood vessels.
Peripheral nerves consist of axons bundled together within an epineurium, or
connective tissue sheath. The collagen here confers ess
ential tensile strength
to the nerve, since axons themselves are quite delicate.
Clinical note: The epineurium gives surgeons something to hold onto and suture through
when reattaching severed nerve ends.
Peripheral nerves are functionally meaningful only in
relation to their connections. All of the axons which travel along
peripheral nerves begin and end somewhere else.
Motor axons originate with cell bodies in the spinal
cord's ventral horn or in the brainstem's motor nuclei
or in peripheral sympathetic or parasympathetic ganglia. Motor
axons terminate at muscles (including smooth muscle along blood vessels)
or glands.
Somatosensory axons begin with a peripheral receptor
(e.g., a Meissner's corpuscle in skin or a muscle-spindle in a muscle).
These sensory axons then travel toward their cell bodies in a dorsal
root ganglion or trigeminal ganglion, and finally terminate at
synapses within the spinal cord or brain stem. (Note that somatosensory
axons are an exception to the rule that axons always conduct impulses away
from the cell body.)
All the cellular nuclei which are obviously visible within a peripheral nerve
belong not to nerve cells but to Schwann
cells or to fibroblasts.
In routine H&E slides, three types of fibrous tissue bear some resemblance to one
another: peripheral nerves, smooth muscle, and collagen.
All three are eosinophilic, and all three contain scattered, elongated nuclei.
Several features may be used to distinguish nerves from smooth
muscle or other fibrous tissue.
- Nerves have a sheath of fibrous connective
tissue, the epineurium, that forms a discrete boundary around
the nerve. Small nerves, and bundles of axons within large nerves,
are also ensheathed by perineurium, including squamous cells (perineural
epithelium) whose intercellular junctions isolate the axons from surrounding
connective tissue.
Clinical note: The epineurium gives surgeons something to hold onto and suture through
when reattaching severed nerve ends.
- Unless specially stained, nerve fibers tend to appear rather pale.
- Neither myelin (which is mostly fat) nor axoplasm
(which is mostly water) is readily stained by H&E.
- Nerves often have a characteristic swirled or wavy texture, because axons
in the nerve tend to twist, like fibers in a string.
- Axons need to be somewhat longer than the nerve within which they
run, so that if the nerve is stretched the axons do not all snap.
- The nuclei found within nerves are mostly Schwann cell nuclei.
- Schwann cell nuclei are usually larger and with less-condensed chromatin
than fibroblast nuclei.
- A small bundle of smooth muscle can resemble
a nerve, but smooth muscle bundles have no sheath. With commonly used stains, smooth muscle
is typically colored more intensely than nerve.
- In longitudinal section, smooth muscle nuclei
typically appear considerably longer than either Schwann or fibroblast
nuclei, and in cross section of well-prepared specimens these nuclei
can usually be seen to reside within the fibers (unlike either Schwann
cell or fibroblast nuclei, which lie alongside the associated fibers).
Note that the texture of peripheral nerves can differ from site to site,
depending on axon size and especially on the proportion of myelinated to unmyelinated
axons. For example, nerves in the tongue, with many large myelinated axons, are much
more obvious than are autonomic nerves in Auerbach's plexus of the gut, where
most axons are smaller and unmyelinated.

 In peripheral nerve cross sections stained for myelin, the myelin
is generally visible as a dark or black frame around each pale myelinated
axon. The typical round shape is often distorted by tissue preparation.
In longitudinal sections containing large myelinated axons, nodes
of Ranvier can sometimes be noticed where the myelin appears to be "pinched."
Seldom can a single axon be followed throughout an entire internode
(i.e., the segment of myelin from one node to the next, about 1-2 mm); the
axon is just not straight enough to remain within the plane of section. Nevertheless,
the length of each internode can be estimated by measuring the total length
of all axons visible in a field of view and dividing by the number of nodes
that appear.
In peripheral nerve cross sections stained for myelin, the myelin
is generally visible as a dark or black frame around each pale myelinated
axon. The typical round shape is often distorted by tissue preparation.
In longitudinal sections containing large myelinated axons, nodes
of Ranvier can sometimes be noticed where the myelin appears to be "pinched."
Seldom can a single axon be followed throughout an entire internode
(i.e., the segment of myelin from one node to the next, about 1-2 mm); the
axon is just not straight enough to remain within the plane of section. Nevertheless,
the length of each internode can be estimated by measuring the total length
of all axons visible in a field of view and dividing by the number of nodes
that appear.
In ordinary H&E stained cross sections of peripheral nerve, myelin
might be visible as a pale unstained halo around a larger axon. Less-than-ideal
fixation often distorts the relationship, so the axon may not be centered
within the halo and the myelin itself easily mistaken for extracellular space.
Many details of peripheral nerves cannot be well-appreciated by light microscopy.
For electron micrographs of peripheral nerves, see the online Electron
Microscopic Atlas of cells, tissues, and organs (the text is in German, but most
figure labels can be deciphered fairly easily).
TOP OF PAGE
SENSORY and MOTOR NERVE ENDINGS associated with PERIPHERAL NERVES
For sensory receptors in skin, see skin innervation.
For sensory receptors associated with muscle, see muscle
innervation. Cell bodies for these and most other somatosensory endings are located in dorsal root ganglia.
For motor endings on skeletal muscle, see muscle
innervation. Cell bodies for these motor endings are the spinal
motor neurons in the anterior horn of the spinal cord.
TOP OF PAGE
Composition of CENTRAL NERVOUS SYSTEM
The organization of the central nervous system is based upon interconnections
across varying distances among billions of individual nerve cells. The
basic principle of neural organization is quite straightforward: Nervous
tissue consists of nerve cells communicating with other nerve cells. This
simple yet fundamental concept can easily become lost in the forest of details
presented in standard textbooks. Here, then, is a brief guide to nervous
tissue, including the classification and nomenclature of nerve cells.
Gray Matter and
White Matter (Cortex, Nuclei, and Fiber Tracts)
 Each
nerve cell has a cell body in one place and an axon
which travels some distance to synapse with the cell bodies and dendrites
of other neurons.
Each
nerve cell has a cell body in one place and an axon
which travels some distance to synapse with the cell bodies and dendrites
of other neurons.
- Regions consisting of cell bodies together with their associated
dendrites and axon terminals are termed gray matter.
- Gray matter located on the surface of the brain is called cortex
(e.g., cortex of the cerebral hemispheres, cortex of the cerebellum).
- Masses of gray matter located deeper in the brain are called nuclei.(e.g.,
brainstem nuclei, nuclei of the thalamus).
- Regions consisting of axons gathered into bundles, to the exclusion
of cell bodies, are called white matter.
- White matter in which all of the axons lie parallel to one another is
called a fiber tract.
- A fiber tract which crosses the midline to connect bilaterally symmetric
structures is called a commissure.
The microscopic appearances of gray matter and white matter may be conveniently
contrasted in a section of spinal cord.
Various stains have variously differential effects on gray and white matter. Note that a popular
neuroanatomical stain (Weigert's stain, commemorating Karl Weigert, b. 1845), used to highlight different brain regions,
colors myelin black. Thus, paradoxically, in many pictures
of the brain, white matter appekars black while gray matter
appears pale.
TOP OF PAGE
 Gray
matter: Where cell bodies and dendrites are common, the gross color
of fixed (dead) brain tissue is gray. Hence we have the term gray
matter. Note that gray matter is not just a place where cell
bodies and dendrites happen to be. Gray matter is the
cell bodies and dendrites.
Gray
matter: Where cell bodies and dendrites are common, the gross color
of fixed (dead) brain tissue is gray. Hence we have the term gray
matter. Note that gray matter is not just a place where cell
bodies and dendrites happen to be. Gray matter is the
cell bodies and dendrites.
- Living gray matter is not gray but rather pink,
due to blood perfusing through very numerous brain capillaries. (The
brain is intensely vascular, with each cubic centimeter of brain tissue
having around 100 square centimeters of capillary endothelial surface area.)
A literary aside: "Gray matter" is colloquially used as an expression for "smarts."
But, Hercule Poirot notwithstanding, there is
no such thing as "little gray cells."
- Cortex is gray matter found on the surface of the
brain. There is cerebral cortex covering
the surface of cerebrum and cerebellar cortex
covering the surface of the cerebellum. But not all gray matter is
cortical.
- A nucleus is a mass of gray matter found deep in
the brain. Such nuclei are not to be confused with nuclei of individual
cells, although neuron cell bodies with their cell-nuclei are found within brain-nuclei
(and not in white matter).
Note that gray matter necessarily contains both the beginnings and endings
of axons, even though the greater portion of many axons' length is contained
within the fiber tracts of white matter. Gray matter is gray not because
it lacks myelin, but because it contains lots of other stuff besides myelinated
axons.
TOP OF PAGE
White matter: Axons from many different neurons often gather
together in large numbers at some distance from their cell bodies. In
such regions, the relatively large amount of myelin confers a white
color, hence, white matter. Myelin is largely fat, which is white
in both living and fixed condition.
NOTE: In many neuroanatomical images, white matter has been stained
black.
Clinical note: White matter is selectively involved in some disorders. See the journal
Science 372:6548 (18 June 2021)
for an essay on white matter per se.
- White matter represents axons going relatively long distances.
It is the stuff of "fiber tracts" or "neural pathways."
- If white matter is cut, the cell body at one end of each
axon is disconnected from its distal axon terminals at the other end.
- In some white matter areas most axons are travelling in
parallel, with all action potentials propagating in the same direction.
For example, most axons in the dorsal columns are ascending, while
those in the cortico-spinal tract are descending.
- But in many other white matter regions of the CNS, adjacent
axons may carry signals in opposite directions or be interwoven in a complex
meshwork. For example, axons in the internal capsule and corpus
callosum crisscross back and forth, interconnecting many different regions
of the cerebral hemispheres.
Although white matter consists of myelinated axons (and unmyelinated
axons as well), myelinated axons are not excluded from gray
matter. Myelinated axons must begin and end somewhere, and that
place is with cell bodies and dendrites of gray matter. Gray matter
just has a lot of other stuff in it besides myelinated axons.
TOP OF PAGE
Sensory Neurons, Motor Neurons, and Interneurons
 Sensory neurons convey sensory information into the central nervous system.
Primary sensory neurons receive their information directly through sense receptors
rather than dendrites. Second-, third- and higher-order sensory neurons
relay information to sequentially higher levels in the brain.
Sensory neurons convey sensory information into the central nervous system.
Primary sensory neurons receive their information directly through sense receptors
rather than dendrites. Second-, third- and higher-order sensory neurons
relay information to sequentially higher levels in the brain.
Primary sensory neurons have their cell bodies located outside the central nervous system.
For example, most somatosensory cell bodies reside in dorsal root ganglia (or in the trigeminal ganglion).
Motor neurons (or motoneurons) convey information out from the central
nervous system to muscles or glands. Lower motor
neurons, located in the ventral horn of the spinal
cord or in motor nuclei of the brainstem, send their motor axons out peripheral
nerves. Upper motor neurons, pyramidal cells
located in the motor cortex, relay information
to the lower motor neurons.
All other neurons are interneurons. They interconnect neurons
with other neurons. Nearly all the nerve cells in the central nervous
system are interneurons. Their axons arise in one region of the CNS
where the cell body resides and end somewhere else (sometimes several other
places). Second order, third order, and higher order sensory neurons can be considered
as ascending interneurons; upper motor neurons can be considered
as descending interneurons.
Synaptic Relays
Information from primary sensory neurons does not reach the highest
levels (the cerebral cortex) directly. Rather it is relayed at least
twice (once in the spinal cord or brain stem, again in the thalamus).
At each relay, incoming (afferent, presynaptic) axons
terminate by synapsing onto the dendrites of the next neurons in the series.
The outgoing axons of these neurons then relay the information to the
next level. At each relay site, some information processing and distribution
can occur, so the information can be altered as it travels upward. Similarly,
muscle commands are relayed downward from motor cortex and other motor
centers to the "final common pathway," the lower motor neurons
of cranial nerve nuclei and the anterior horn of the spinal
cord.
Because each relay occurs at synapses onto dendrites and cell bodies of
the next neurons in the pathway, each relay is associated with gray matter.
Conversely, every gray matter region (nucleus or cortex) is associated
with relaying information from one set of axons (the afferent
axons that enter the region in question) to another (the efferent
axons that leave the region).
Sometimes it is sufficient just to know the beginning and ending points
of an entire pathway. Other times knowing how far the neurons of each
relay extend will be necessary to determine the site or effects of a lesion.
TOP OF PAGE
Afferent and Efferent Axons
All gray matter regions of the brain, both cortex and nuclei, are associated
with afferent ("input") and efferent ("output") axons. Afferent
axons enter the region from somewhere else (i.e., the cell body is located
some distance away). Efferent axons arise from cell bodies within the region
and leave the region to go somewhere else. Thus every long-distance
axon is both efferent (with respect to its source, the location of its cell
body) and afferent (with respect to its destination).
The terms "afferent" and "efferent" are relational terms. Neither can be
used precisely without specifying a region of reference. For example, "cortical afferents"
provide input to cortex; they may be efferents from thalamus or efferents
from some other cortical area.
Ascending and Descending Pathways
"Ascending" and "descending" refer to directions along
the neural axis. These terms may often correlate with "afferent"
and "efferent," at least when the reference is high, like
cortex. (In fact, "afferent" and "efferent" are sometimes used as synonyms
of "ascending" and "descending," respectively. But they also have a
relational meaning, defined above. "Ascending" and "descending"
are also closely associated with "sensory" and "motor," respectively. But
both sensory and motor information can be passed up, down, and sidewise, so
these words should not be carelessly interchanged.
TOP OF PAGE
Long-Axon and Short-Axon Neurons
Gray matter typically contains both many short-axon neurons and a smaller
number of long-axon neurons.
Long-axon neurons (also called principal cells, projection neurons, or
Golgi type 1 neurons) have the largest and most conspicuous cell bodies in a particular region of gray
matter. These cells generally have very long axons which leave the local region
to project elsewhere, usually traveling within some white matter fiber tract.
These long axons may extend for appreciable distances,
up to many centimeters. Long-axon neurons are responsible
for communicating with other brain regions. Every parcel of gray matter
has a class of long-axon neurons; otherwise information would come in but
never go out. The axons of a region's long-axon neurons are by
definition identical with the region's efferent axons.
- Such large, long-axon cells may have specific names like
pyramidal cells (the output cells of the
cerebral cortex) or Purkinje cells (the output
cells of the cerebellar cortex).
- A large cell body is probably necessary to support the
cytoplasmic volume and membrane surface area of an extremely long axon. (For
exercise, you might try estimating the volume of axoplasm in an axon 5 Ám in diameter and
ten centimeters long. Compare that result with the volume of cytoplasm
in a cell body 50 Ám in diameter.)
The study of neuroanatomy is largely the study of the axonal projections
of long-axon cells.
Historical note: See
"The study of neuroanatomy" above.
Short-axon neurons (also called intrinsic neurons, local
interneurons, or Golgi type 2 neurons) have relatively short axons that do not leave the immediate neighborhood.
- Short-axon neurons are presumably responsible for integrating
information from diverse sources. ("Integrating" is the
jargon term for what nervous tissue does when it transforms information,
or thinks. The word "integration" provides a convenient name for a
process that is otherwise
poorly understood.)
- Cell bodies of short-axon neurons are often small and
numerous. They come in various shapes, and are given names according
to the region where they are found (see neuron names,
below).
- Most so-called granule cells and stellate cells
are local interneurons.
In most regions, long-axon cells are much better understood than intrinsic
cells. Long axons provide opportunity for researchers to record and
interfere with neuronal output. Cells with short axons are much more
difficult to manipulate.
Historical note: The terms "Golgi type 1" and "Golgi type 2" commemorate
pioneering neuroscientist Camillo Golgi (b. 1843).
TOP OF PAGE
Nerve cell types and nerve cell names
Unlike many other cell types in the body (e.g., epidermal keratinocytes or skeletal
osteocytes), nerve cells are not all equivalent to one another. Neurons
in one region are structurally and functionally different from those in other
regions, with different sources of input, different destinations for output,
different patterns of dendritic branching, different neurotransmitters, etc.
Similar diversity is found even within a given region (e.g.,
cerebral cortex and cerebellar
cortex, above). The result is a tremendous variety, with specific names for quite
a few distinctive nerve cell types.
Indeed, in many animals certain neurons can each be identified
as a unique
individual cell, with features that distinguish that one particular cell from any other cell
in the same animal. Such identifiable
neurons can be consistently recognized from specimen to specimen, sometimes even in specimens
from related species.
Historical note: The great variety of nerve
cells was famously observed and described by
Ramón y Cajal from the late 1800s through the early 1900s. "So varied were the cells'
structures in the human cortex -- some look like stars, others like bird's nests, baskets,
or spindles -- that Cajal divided them into dozens of subtypes. He argued that the diversity
of the cells, which he called the 'butterflies of the soul,' was the key to
higher cognition" (quote from Science
349:575, 7 Aug 2015).
Recent research: In 2021, the journal
Nature 598:66 (6 October 2021) provided an initial progress report on "a multimodal cell census and atlas
of the mammalian primary motor cortex as the initial product of the BRAIN Initiative Cell Census Network." In
this report, "cells grouped according to their transcriptomes tend to share other features, such as location, shape,
and electrical activity. That finding 'provides strong validation to the molecularly defined cell types... For
the human motor cortex, 127 such types emerged'" (quote from
Science daily news, 7 October 2021). Note that those 127 cell types were all from one single -- and rather small -- brain region,
the primary motor cortex.
This work on the BRAIN Initiative Cell Census continues apace, with multiple articles published on Oct. 13, 2023, in a special issue of
Science. For a nice news media
report by Carl Zimmer, see The New York
Times, 12 Oct. 2023: "Researchers identified some 3,300 types of brain cells, an order of magnitude more than was
previously known, and have only a dim notion of what most of them do."
Nerve cells in the brain of Drosophila have been much more thoroughly mapped
[Nature 634: 139-152, 2024;
New York Times article by Carl Zimmer, October 2, 2024],
with 140,000 neurons of 8,453 cell types.
Proper synaptic connections between specific neurons is guided by a "barcode" of cell-surface proteins; see
Science 385:370 (26 July 2024).
As a result of this diversity of nerve cell types, neurohistology is burdened by a profusion
of nerve cell names. Every nerve cell can be classified according to its place
within the general organization of nervous tissue (above). But each
nerve cell also belongs to a unique population with a particular role in the
information processing of the brain.
Examples of a few nerve cell types, familiar from many textbook accounts, are described below.
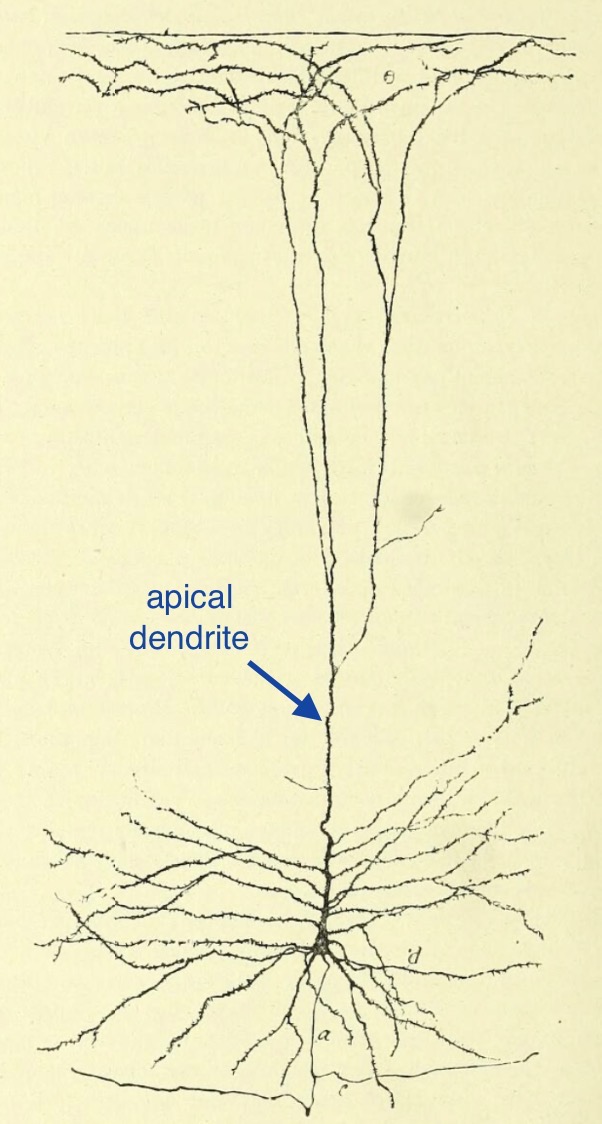
 Pyramidal
cells are the efferent (long-axon) cells of the cerebral
cortex. The name refers to the shape of the cell body as seen in
standard sections perpendicular to the cortical surface. The apex of
the pyramid points toward the cortical surface. A large apical dendrite
extends farther upward toward that surface, while other dendrites arise from
the corners and sides of the pyramid. The axon extends down into white matter (the internal capsule) from the base of the pyramid. Most pyramidal
cells project association fibers to other cortical regions and/or to deeper
nuclei of the brain. (Classic illustrations of these cells were done
by Ramón y Cajal).
Pyramidal
cells are the efferent (long-axon) cells of the cerebral
cortex. The name refers to the shape of the cell body as seen in
standard sections perpendicular to the cortical surface. The apex of
the pyramid points toward the cortical surface. A large apical dendrite
extends farther upward toward that surface, while other dendrites arise from
the corners and sides of the pyramid. The axon extends down into white matter (the internal capsule) from the base of the pyramid. Most pyramidal
cells project association fibers to other cortical regions and/or to deeper
nuclei of the brain. (Classic illustrations of these cells were done
by Ramón y Cajal).
 The giant Betz cells (named for Vladimir Betz, b. 1834)
are extremely large pyramidal cells of the motor
(precentral) cortex. These pyramidal cells comprise some of the
upper motor neurons. Axons from these cells descend in the corticospinal
tract, or pyramidal tract, to synapse with lower motor neurons. The
exceptionally large size of Betz cell bodies is presumably associated with their need to sustain
extremely long axons.
The giant Betz cells (named for Vladimir Betz, b. 1834)
are extremely large pyramidal cells of the motor
(precentral) cortex. These pyramidal cells comprise some of the
upper motor neurons. Axons from these cells descend in the corticospinal
tract, or pyramidal tract, to synapse with lower motor neurons. The
exceptionally large size of Betz cell bodies is presumably associated with their need to sustain
extremely long axons.
Stellate cells are intrinsic neurons named for their star-like shape,
which results from dendrites arising in many directions. The name is
descriptive but relatively non-specific. Thus stellate cells of the
cerebral cortex are not the same as stellate
cells of the cerebellar cortex.
Horizontal cells are intrinsic neurons whose dendrites and local
axons tend to be confined within a layer parallel to a surface. Again
the name is descriptive rather than specific. Thus horizontal cells
in the cerebral cortex are not the same
as horizontal cells in the retina.
 Granule
cells are intrinsic neurons which are both very small and very numerous,
like grains of sand. Once again, this name is descriptive but non-specific
unless additionally qualified. For example, cerebellar granule cells
are a very specific class of short-axon cells in one layer of the cerebellar
cortex (a layer called, unimaginatively, the "granule cell layer").
Granule
cells are intrinsic neurons which are both very small and very numerous,
like grains of sand. Once again, this name is descriptive but non-specific
unless additionally qualified. For example, cerebellar granule cells
are a very specific class of short-axon cells in one layer of the cerebellar
cortex (a layer called, unimaginatively, the "granule cell layer").

 Purkinje cells (named after Johann Purkinje,
b. 1787) are the efferent (long-axon) cells of the cerebellar
cortex. Purkinje cells have such impressive dendritic arborization,
that images of these cells (often from the classic
illustrations of Ramon y Cajal) are commonly featured in
textbooks.
Purkinje cells (named after Johann Purkinje,
b. 1787) are the efferent (long-axon) cells of the cerebellar
cortex. Purkinje cells have such impressive dendritic arborization,
that images of these cells (often from the classic
illustrations of Ramon y Cajal) are commonly featured in
textbooks.
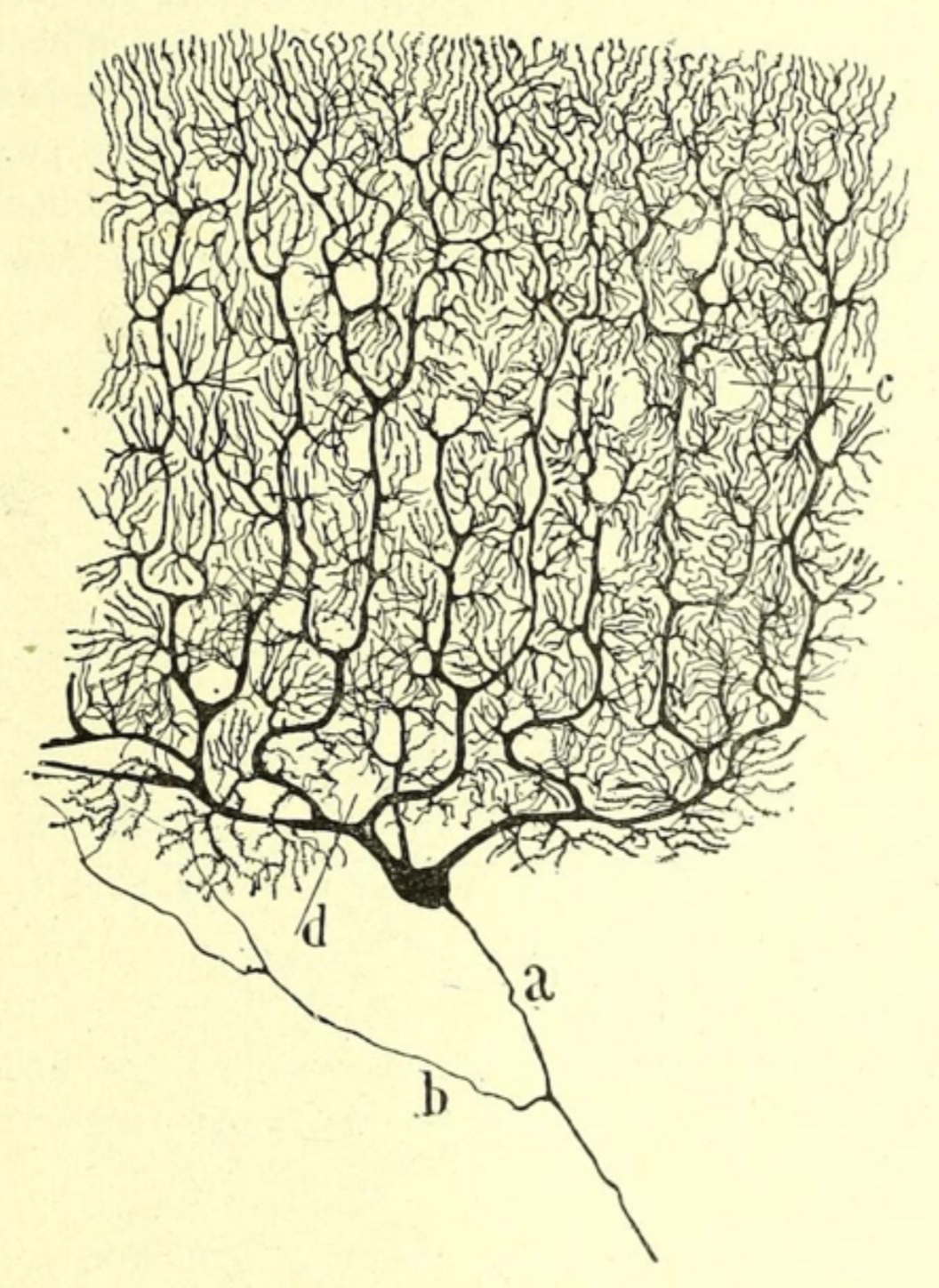
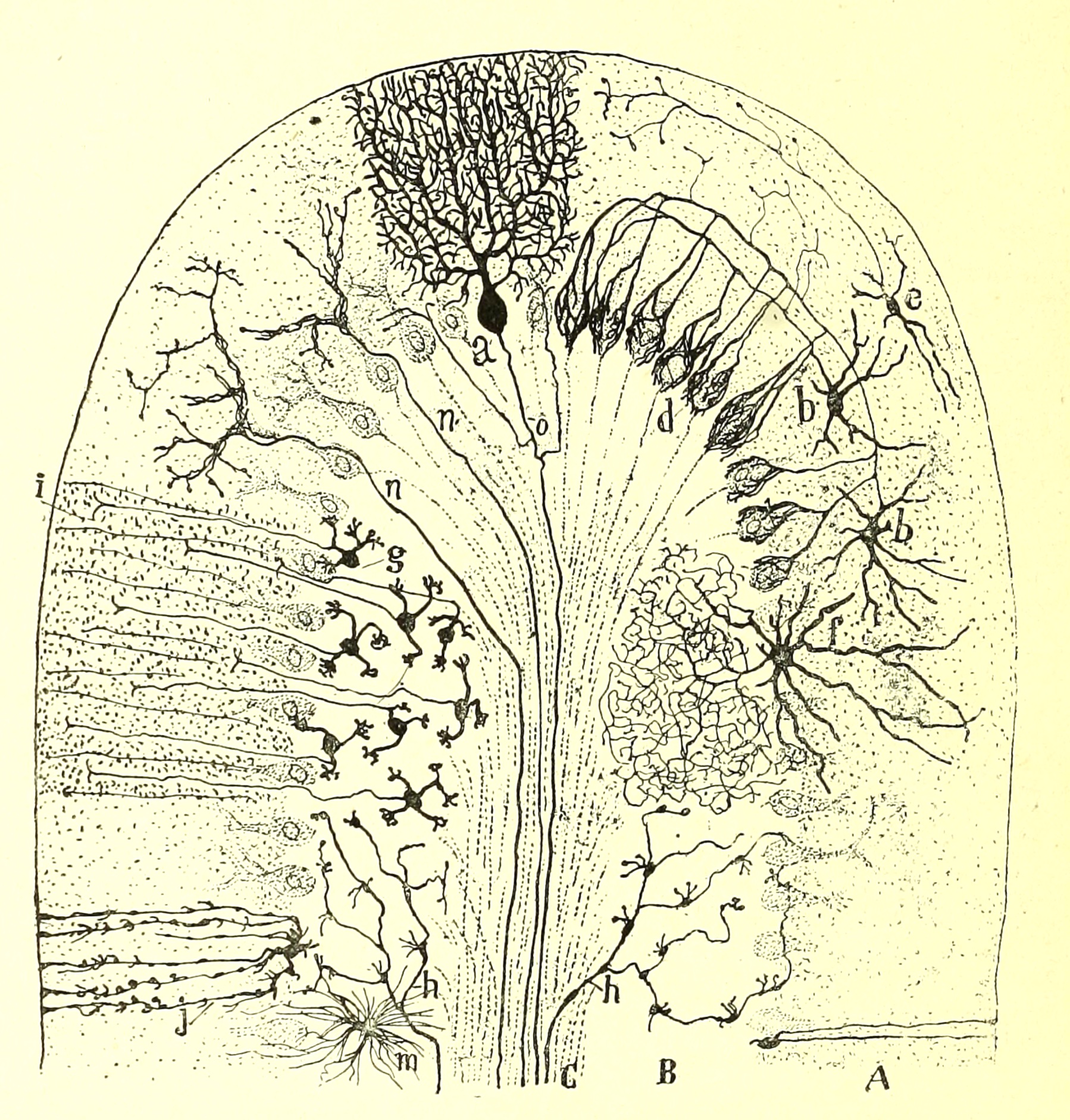 The synaptic input of
Purkinje cells comes largely from the cerebellar parallel fibers (which are
the axons of the extremely numerous local interneurons, the cerebellar granule
cells). Purkinje cells also receive potent synapses directly from one
class of cerebellar afferent fibers, the "climbing fibers." (Another
class of cerebellar afferents, the "mossy fibers," provide input to the intrinsic
granule cells. In addition to Purkinje cells and granule cells, the
cerebellar cortex also contains stellate cells, basket cells, and Golgi cells.)
The synaptic input of
Purkinje cells comes largely from the cerebellar parallel fibers (which are
the axons of the extremely numerous local interneurons, the cerebellar granule
cells). Purkinje cells also receive potent synapses directly from one
class of cerebellar afferent fibers, the "climbing fibers." (Another
class of cerebellar afferents, the "mossy fibers," provide input to the intrinsic
granule cells. In addition to Purkinje cells and granule cells, the
cerebellar cortex also contains stellate cells, basket cells, and Golgi cells.)
[See your favorite neuro text (e.g., Kandel et al.,
4th ed., pp. 835 ff) for elegant details of neural circuitry in the cerebellar
cortex.]

Spinal motor neurons have the largest nerve cell bodies in the ventral
horn. These cells are also called "lower motor neurons,"
or just "motor neurons" (since upper moter neurons are properly
called interneurons). Axons of these cells extend through ventral (anterior)
roots into peripheral nerves, and hence to motor end plates on muscle fibers. Each
muscle fiber of skeletal muscle is innervated by
a single spinal motor neuron. Each spinal motor neuron innervates one
or more muscle fibers; the number of muscle fibers per axon is related to
the fineness of motor control -- e.g., one-to-one for eye muscles, many-to-one
for large postural muscles. A single spinal motor neuron and all the
muscle fibers innervated by its axon are called a motor unit. (In the cartoon
diagram to right, a spinal motor neuron is represented extending into the figure's right leg to
innervate a calf muscle. The upper neuron in this cartoon, whose cell body is located in
the head, represents an upper motor neuron, or pyramidal cell, of
motor cortex.)
Dorsal root ganglion neurons (which are primary somatosensory neurons) are among
the most distinctively shaped of our nerve cells. Their cell bodies are peculiarly located in
dorsal root ganglia alongside the spinal cord. A single process leaves each
cell body, then bifurcates into two very long branches; both branches together comprise a single functional axon. The distal branch serves one of many peripheral touch or proprioceptor sensory nerve endings. The proximal branch enters the spinal cord and contributes to one of the
ascending afferent tracts, with some interneuronal connections along the way.
(In the cartoon diagram to right, a dorsal root ganglion neuron is represented extending from the
figure's left toe up to the figure's neck.)
The above are only examples, from classical textbook literature.
Every region of the central nervous system
contains many distinct neuronal cell types, most of which are appreciated
only by the research specialist. But a few such examples are worth knowing,
if only because they are a part of familiar scientific knowledge and because
they hint at the complexity of neural organization.
Note that in every region of the brain, the long-axon cells are directly associated with
significant neuroanatomical pathways (output), while detailed knowledge of the intrinsic
cells generally has little clinical importance -- at least in our current state
of ignorance.
TOP OF PAGE
NERVOUS TISSUE PATHOLOGY.
Washington
University hosts an excellent web resource for neuromuscular
pathology.
Within this site, you can find Differential Diagnosis for myopathy
and neuropathy, and much
more.
Chapter 18 in the 3rd edition of Kandel et al. (1991) is an
excellent resource for understanding the responses of nervous tissue to injury.
The newer, 4th edition of Kandel et al. (2000) offers a much sketchier
account in Chapter 55 (pp. 1108 ff).
WebPath
also offers some examples of nervous system pathology, see WebPath
CNS Pathology Index and WebPath
CNS Degenerative Diseases.
For example:
- Spinal motor neurons are lost in amyotrophic lateral
sclerosis (WebPath,
gross; WebPath,
neuron loss; WebPath,
gliosis).
- Alzheimer's disease (WebPath)
is accompanied by cortical changes (WebPath
gross, WebPath,
WebPath,
WebPath,
WebPath,
WebPath,
WebPath). Each of these links offers a different illustration.
- Inflammation, as in meningitis (WebPath
), presents infiltration of leukocytes into regions, such as CSF, where
they are normally absent.
- Multiple sclerosis (WebPath,
WebPath
w/ MRI ) involves patchy loss of CNS myelin, apparently caused either
by autoimmune destruction of oligodendroglia or by malfunction of oligodendroglia
with immune destruction as a secondary effect (Science 308:778, 2005).
Resulting loss of conduction can cause a variety of neurological problems.
- "Microscopically, the caudate nucleus in Huntington's
disease demonstrates loss of neurons along with gliosis" (WebPath,
gross; WebPath,
microscopic).
- Parkinson's disease (WebPath
) involves loss of the pigmented neurons in the brainstem which give substantia
nigra ("black substance") its distinctive appearance.
- Brain tissue in CJD or Creutzfeldt-Jakob disease
(WebPath,
WebPath,
WebPath)
is characterized by microscopic vacuoles (hence, "spongiform encephalopathy")
and plaques.
- Schwann cells can form tumors called schwannomas
(see WebPath: MRI,
gross,
dissection,
microscopic low
X, high
X).
TOP OF PAGE
Comments and questions: dgking@siu.edu
SIUC / School
of Medicine / Anatomy / David
King
https://histology.siu.edu/ssb/neuron.htm
Last updated: 9 September 2025 / dgk
 Neurons
and Support Cells
Neurons
and Support Cells 





 Nerve cells come in extreme variety. In every region
of the brain are several different nerve cell types, each distinguished
by its own characteristic soma size, dendritic shape, source of synaptic
input, destination of axonal output, and chemistry (
Nerve cells come in extreme variety. In every region
of the brain are several different nerve cell types, each distinguished
by its own characteristic soma size, dendritic shape, source of synaptic
input, destination of axonal output, and chemistry (



 To
visualize myelin formation:
To
visualize myelin formation:
 A
Schwann cell is illustrated with brown
cytoplasm.
A
Schwann cell is illustrated with brown
cytoplasm.
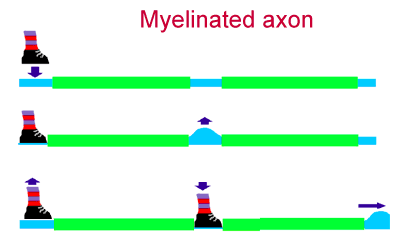














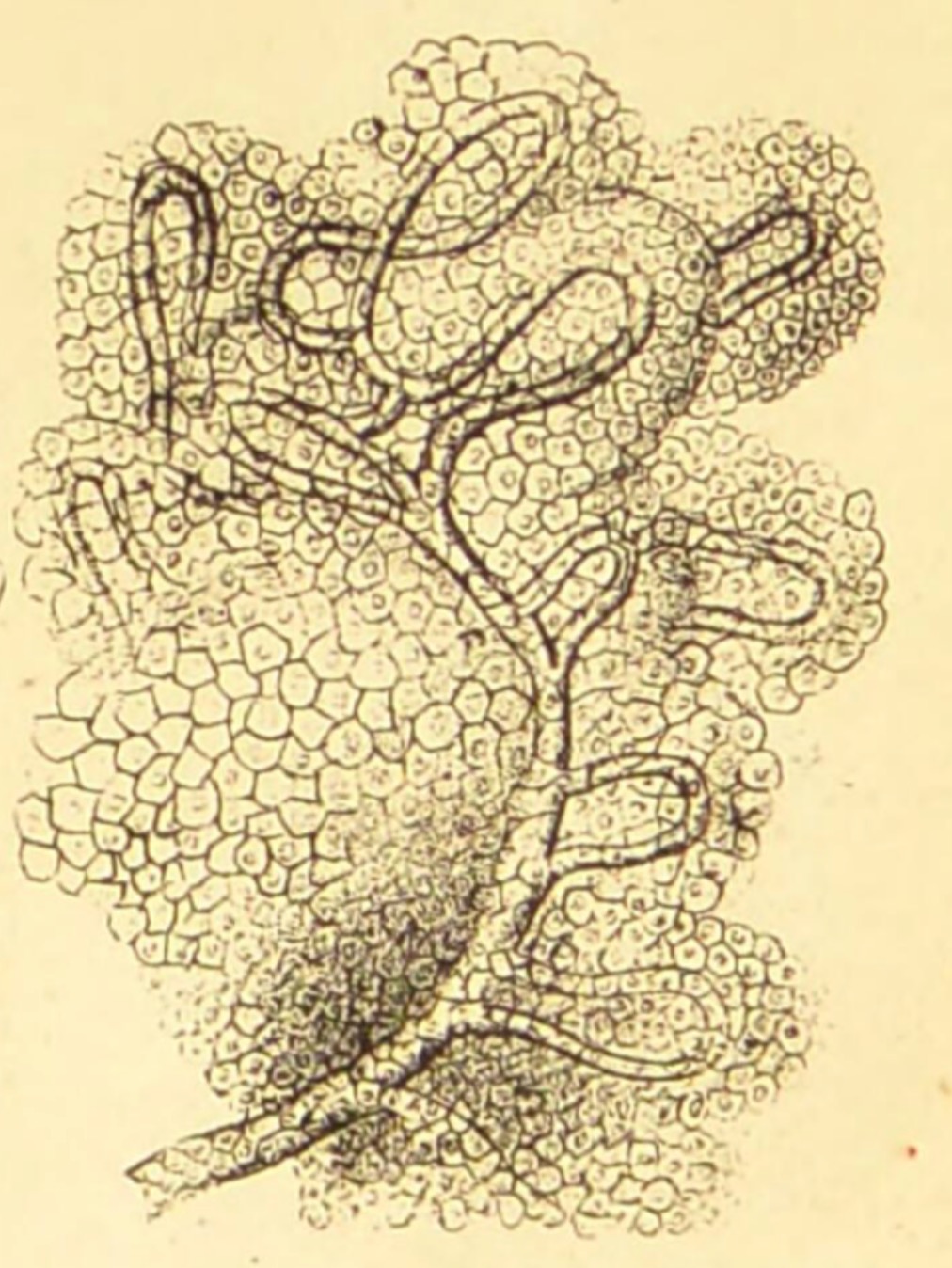


















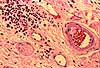
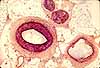
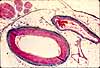
 Sensory neurons convey sensory information into the central nervous system.
Primary sensory neurons receive their information directly through sense receptors
rather than dendrites. Second-, third- and higher-order sensory neurons
relay information to sequentially higher levels in the brain.
Sensory neurons convey sensory information into the central nervous system.
Primary sensory neurons receive their information directly through sense receptors
rather than dendrites. Second-, third- and higher-order sensory neurons
relay information to sequentially higher levels in the brain. 


